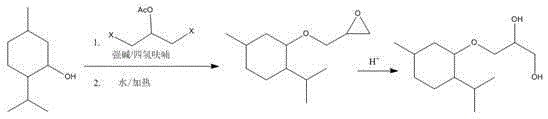
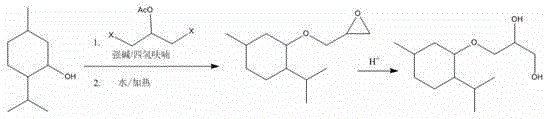
A kind of synthetic method of 3-l-menthoxypropane-1,2-diol
A technique for the synthesis of menthoxypropane and its synthesis method, which is applied in the preparation of ethers from alkylene oxides, chemical instruments and methods, and the preparation of organic compounds. Mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1, under nitrogen protection, menthol (156g, 1mol, 1eq) was added to a three-necked reaction flask filled with 300ml of anhydrous tetrahydrofuran, and when the internal temperature was lowered to 0-10°C, 60% NaH (48g, 1.2mol, 1.2eq), after the addition is complete, slowly raise the temperature to room temperature, stir at room temperature for 30-50min and then lower the temperature to 0-10°C, add 1,3-dichloroglycerol acetate dropwise (162.45g, 0.95mol, 0.95eq) tetrahydrofuran solution, dropwise completed, the temperature rose to 60 o C. Stir for 1-2 hours, drop to 0-15°C, add a certain amount of water dropwise, and add a catalytic amount of tetrabutylammonium iodide (3.2g), and raise the temperature to 60-70°C and stir for 3h. After the reaction is completed , extracted three times with n-hexane, 500ml each time, combined the three extracted organic layers, washed each 100ml with water and brine, washed the organic layer once, separated, dried, and distilled n-h...
Embodiment 2
[0014] Example 2, under nitrogen protection, menthol (300g, 1.92mol, 1eq) was added to a three-necked reaction flask containing 500ml of anhydrous tetrahydrofuran, and when the internal temperature was lowered to 0-10°C, 60% NaH (153.8 g, 3.84mol, 2eq), after the addition is complete, slowly raise the temperature to room temperature, stir at room temperature for 30-50min and then lower the temperature to 0-10°C, drop in 1,3-dichloroglycerolacetic acid Ester (328g, 1.92mol, eq) tetrahydrofuran solution, after dropping, raise the temperature to 60°C, stir for 1-2 hours, then drop to 0-15°C, drop a certain amount of water, and add a catalytic amount of tetrabutyl Ammonium iodide (6g, 2%), raise the temperature to 60-70°C and stir for 3h. After the reaction is completed, extract three times with n-hexane, 500ml each time, combine the three extracted organic layers, wash once with 200ml each of water and brine The organic layer was separated and dried, and the n-hexane and tetrahyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com