A method for synthesizing aminopyridine from pyridine base mixture and its separation and purification method
An aminopyridine and mixture technology, which is applied to the synthesis of chemical intermediates and the synthesis of aminopyridines from pyridine base mixtures, can solve problems such as difficult separation of mixtures, and achieve the effects of avoiding environmental pollution, high income and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
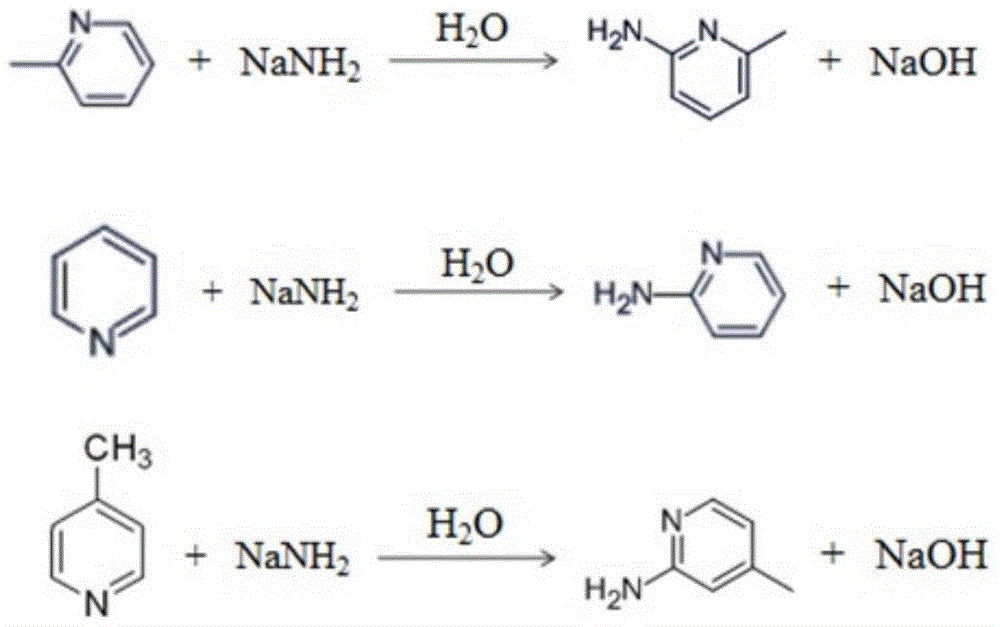
[0037] Such as figure 1 Shown, a kind of method is synthesized aminopyridine by pyridine base mixture, and its step is:
[0038] (a) Xylene (does not contain ferric ion and water) is added in the reaction flask, then the pyridine base mixture that is made up of 2-picoline, pyridine and 4-picoline is added in the reaction flask through a constant pressure funnel and stirred evenly, Wherein the mass ratio of xylene to the pyridine base mixture is 2.5:1, 2-picoline accounts for 90% of the total mass, pyridine is 6%, and 4-picoline is 4% in the pyridine base mixture;
[0039] (b) Slowly heating the reaction flask in step (a) to 80° C. for reflux dehydration until no water droplets are generated in the reaction flask;
[0040] (c) After the dehydration reaction in step (b) is finished, it is cooled to room temperature, and sodium amide is added (addition speed is 10g / min) into the reaction flask and stirred evenly, and then micro-reflux reaction is carried out, wherein sodium amid...
Embodiment 2
[0051] A method for synthesizing aminopyridine from a pyridine base mixture, the steps are:
[0052] (a) Xylene (does not contain iron ion) is added in the reaction flask, then the pyridine base mixture that is made up of 2-picoline, pyridine and 4-picoline is added in the reaction flask through a constant pressure funnel and stirred evenly, wherein two The mass ratio of toluene to the pyridine base mixture is 2:1, and in the pyridine base mixture, 2-picoline accounts for 82% of the total mass, pyridine is 11%, and 4-picoline is 7%;
[0053] (b) Slowly heating the reaction flask in step (a) to 100° C. for reflux dehydration until no water droplets are generated in the reaction flask;
[0054] (c) After the dehydration reaction in step (b) is finished, it is cooled to room temperature, and sodium amide is added in the reaction flask with a speed of 15g / min and stirred evenly, and then micro-reflux reaction is carried out, wherein the mass of sodium amide and pyridine base mixtu...
Embodiment 3
[0065] A method for synthesizing aminopyridine from a pyridine base mixture, the steps are:
[0066] (a) Xylene (does not contain iron ion) is added in the reaction flask, then the pyridine base mixture that is made up of 2-picoline, pyridine and 4-picoline is added in the reaction flask through a constant pressure funnel and stirred evenly, wherein two The mass ratio of toluene to the pyridine base mixture is 3:1, 2-picoline accounts for 55% of the total mass, 28% of pyridine, and 17% of 4-picoline in the pyridine base mixture;
[0067] (b) Slowly heating the reaction flask in step (a) to 135° C. for reflux dehydration until no water droplets are produced in the reaction flask;
[0068] (c) After the dehydration reaction in step (b) is finished, it is cooled to room temperature, and sodium amide is added in the reaction flask with a speed of 20g / min and stirred evenly, and then micro-reflux reaction is carried out, wherein the mass of sodium amide and pyridine base mixture T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com