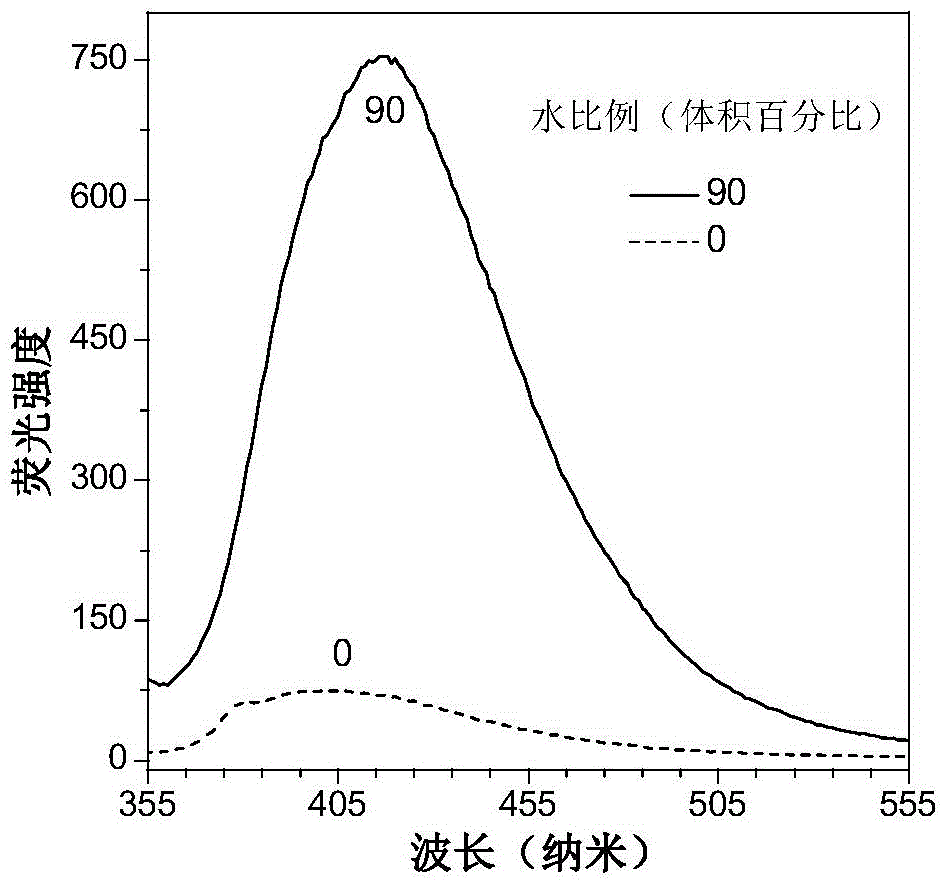
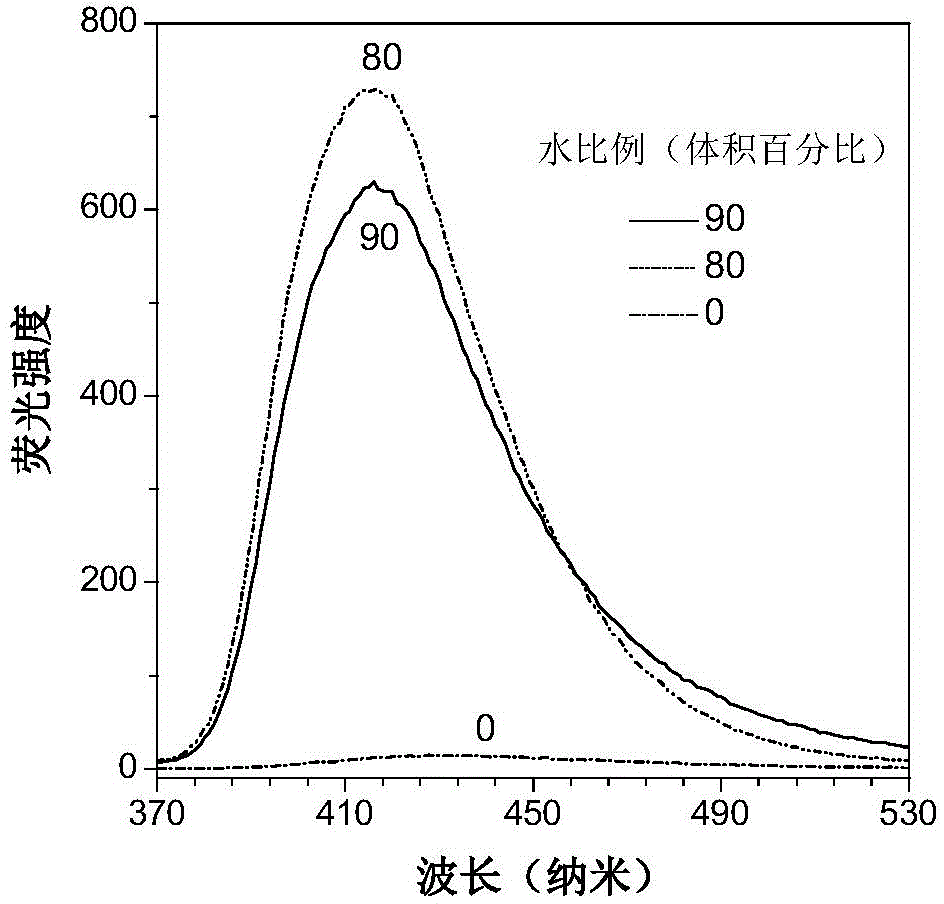
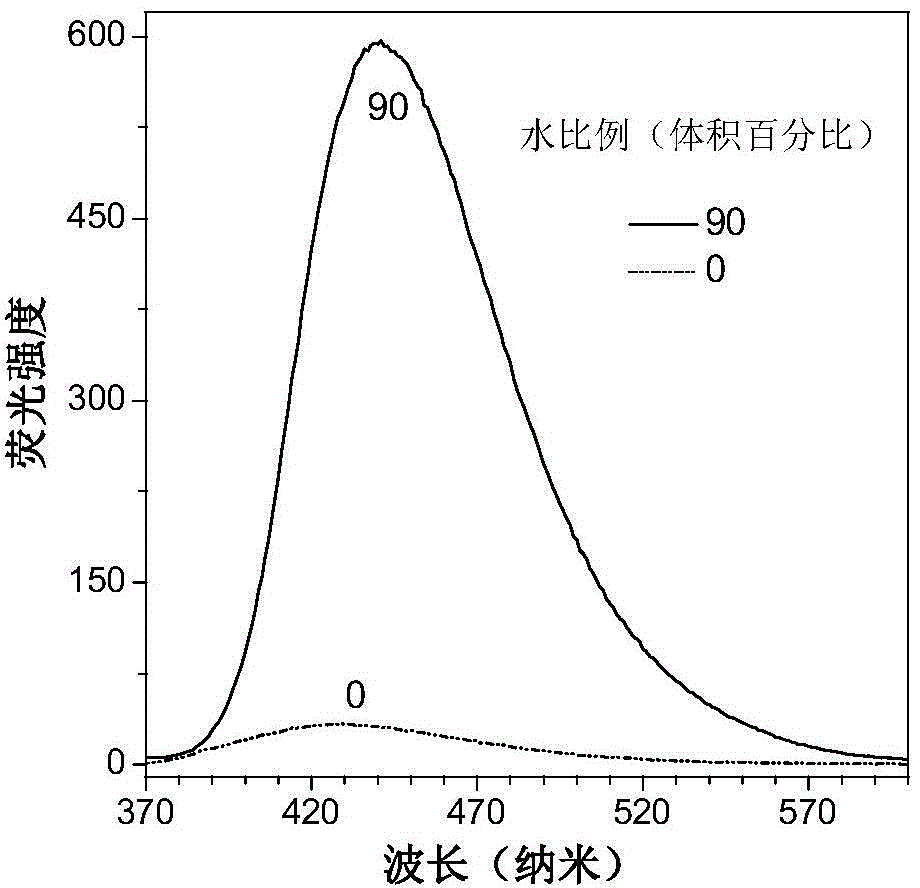
Tetrapherylpyrazine low-molecular-weight derivate, tetrapherylpyrazine polymer and aggregation-induced emission material
A tetraphenylpyrazine and small molecule technology, applied in the field of organic/polymer luminescent materials, can solve the problems of structure destruction, complicated HPS synthesis steps, harsh conditions, etc., and achieve the effects of good stability, easy derivation, and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Synthesis of tetraphenylpyrazine (compound shown in formula 1-1)
[0071]
[0072] Method one: synthetic method references (D.Davidson, M.Weiss and M.Jelling, J.Org.Chem.1937,2,328), in 50mL round bottom flask, add 2.12g benzoin (10mmol), 1.45ml acetic anhydride ( 15mmol), 2.32g ammonium acetate (10mmol) and 10mL acetic acid. The reaction was refluxed in acetic acid for 3.5 hours, the temperature was lowered and suction filtered, and the filter residue was recrystallized in acetic acid to obtain white needle-like crystals, yield: 33.9%. The characterization data of the product are as follows:
[0073] 1 H NMR (500MHz CDCl 3 ): δ(TMS,ppm)7.65(m,8H),7.33(m,12H).
[0074] 13 C NMR (125MHz, CDCl 3 ): δ (TMS, ppm) 148.5, 138.5, 129.9, 128.7, 128.3.
[0075] HRMS (MALDI-TOF): m / z 384.1664 ([M] + ), calcd for C 28 h 20 N 2 384.1626.
[0076] Method 2: Synthetic method References (J.A.Katzenellenbogen, et al., Bioorg.Med.Chem., 2003, 11, 629): In a 50mL round bot...
Embodiment 2
[0079] Synthesis of Tetraphenylpyrazine Derivatives (Compounds Shown in Formula 1-2)
[0080]
[0081] The synthesis method is similar to the method 1 in Example 1 of this patent, the difference is that the raw materials are changed accordingly, white needle-like crystals, yield: 20%. Product characterization data are as follows:
[0082] 1 H NMR (500MHz CDCl 3 ): δ(TMS,ppm)7.61(d,8H),6.86(d,8H),3.82(s,12H).
[0083] 13 C NMR (125MHz, CDCl 3 ): δ (TMS, ppm) 159.9, 146.8, 131.1, 113.7, 55.3.
[0084] HRMS (MALDI-TOF): m / z 504.2039 ([M] + ), calcd for C 32 h 28 N 2 o 4 504.2049.
[0085] The AIE performance of the compound obtained in this example is similar to that of the compound in Example 1.
Embodiment 3
[0087] Synthesis of Tetraphenylpyrazine Derivatives (Compounds Shown in Formula 2-2)
[0088] (1) Synthesis of 1-(4-bromophenyl)-2-acetophenone
[0089]
[0090] Synthetic method reference (C.Cheng, et al., Org.Lett.2010,12,1736): 10g 4-bromophenylboronic acid (50mmol), 1.32g Ni(dppe)Cl were added to a 250mL round bottom flask 2 (2.5mmol) and 5.1gZnCl 2 (37.5 mmol). The reaction system was vacuumed and replaced with nitrogen three times, and then 75mL of 1,4-dioxane, 2.9mL of phenylacetonitrile (25mmol) and 0.45ml of water (25mol) were injected into it, and the reaction was maintained at 80°C for 8h. After the reaction was completed, the filter residue was washed three times with tetrahydrofuran, and the filtrate was spin-dried. The crude product was purified by silica gel column chromatography (petroleum ether / ethyl acetate = 20:1) to obtain a white solid with a yield of 74.4%. The characterization data of the product are as follows:
[0091] 1 H NMR (500MHz DMSO-d 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com