Synthesis method of solifenacin succinate
A technology of solifenacin succinate and synthesis method, which is applied in the field of synthesis of solifenacin succinate, and can solve the problems of increasing the risk of raw material storage and transportation, raw material cost, unfavorable industrialized safe production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
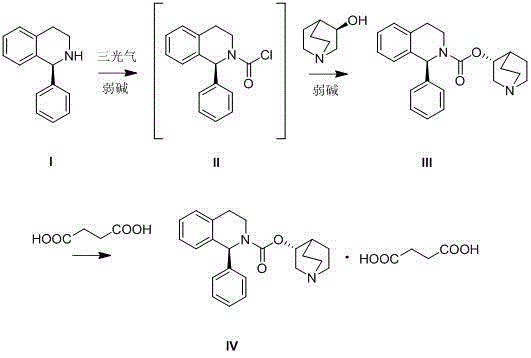
[0033] Dissolve 2.6 g of triphosgene in 40 mL of xylene, cool to 0°C, and slowly add (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline ( I ) 4g, 3.3g of 4-dimethylaminopyridine dissolved in 40mL of xylene solution. After the dropwise addition was completed, it was raised to room temperature to continue the reaction for 3 h, filtered, and the resulting filtrate contained unisolated (S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-formyl chloride ( II ).
[0034] Add 2.8 g of (R)-3-quinuclidinol, 4.8 g of 4-dimethylaminopyridine, and 40 mL of xylene to the solution obtained in the previous step, raise the temperature to 140° C., and stir for 30 h. After cooling to room temperature, add 150 mL of saturated saline, separate the layers, acidify the organic layer with 10% hydrochloric acid, extract with water, then alkalinize with saturated potassium carbonate solution until the pH is approximately equal to 10, extract with ethyl acetate, anhydrous magnesium sulfate Drying, spin-drying solve...
Embodiment 2
[0037] Dissolve 15g of triphosgene in 200mL of xylene, cool to 0°C, add dropwise (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline ( I ) 25g, 20g of 4-dimethylaminopyridine dissolved in 250mL of DMF to form a solution. After the dropwise addition was completed, the reaction was continued at room temperature for 3 h. Add 1 L of water to the reaction liquid in batches to wash and separate the liquids. The organic layer is washed with saturated brine, dried over anhydrous magnesium sulfate, and spin-dried to obtain 28 g of a light yellow oil, which is (S)-1-phenyl-3 , 4-dihydro-1H-isoquinoline-2-formyl chloride ( II)Crude.
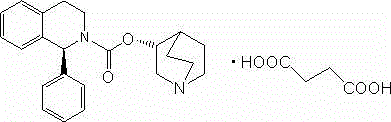
[0038] Gained (S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-formyl chloride ( II ) crude product, 16g of (R)-3-quinuclidinol, and 30g of 4-dimethylaminopyridine were added into 500mL of DMF, the temperature was raised to 140°C, and the reaction was stirred for 30h. After cooling to room temperature, add 500 mL of ethyl acetate and 500 mL of water each. After sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com