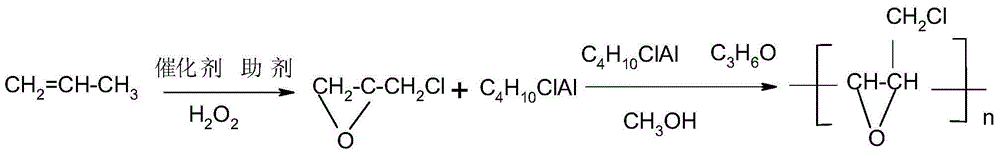Method for synthesizing polyepichlorohydrin
A technology of polyepichlorohydrin and epichlorohydrin, which is applied in the field of synthesis of polyepichlorohydrin, and can solve cumbersome problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] In the 100mL four-necked flask, drop into the chloropropane of 30g and the catalyst of 6.5g (phosphotungstopolyacid / TiO 2 ), then add 10mL of hydrogen peroxide and 5g of diamine hydrogen phosphate under stirring state, and react for 6 hours under reflux of chloropropane. After the reaction was completed, the system was separated into oil and water phase suspensions after standing for 1 hour; the oil layer was separated by distillation to obtain 23.36 mL of epichlorohydrin for future use. Then add 5g of diethylaluminum chloride and 50ml of anhydrous diethyl ether dried over molecular sieves to a three-necked flask equipped with a stirring bar, and stir for 10 minutes at room temperature, cool the stirred solution to 0°C with ice-salt water, and Add 10 mL of propylene oxide dropwise. By controlling the dropping rate to 1 drop / sec and maintaining the temperature at 5 °C. After reacting for 30 minutes, the volatile matter was extracted in time, and then 20 mL of pre-prepa...
example 2
[0025] Put 30g of chloropropane and 6.5g of catalyst (phosphotungstic heteropolyacid / Ti02) into a 100mL four-neck flask, then add 10mL of hydrogen peroxide and 5g of diamine hydrogen phosphate under stirring, and react under reflux of chloropropane for 6 hours. After the reaction was completed, the system was separated into oil and water phase suspensions after standing for 1.5 hours; the oil layer was separated by distillation to obtain 23.36 mL of epichlorohydrin for future use. Then add 5g of diethylaluminum chloride and 50ml of anhydrous diethyl ether dried by molecular sieves to the three-necked flask equipped with a stirrer, and stir at room temperature for 10 minutes, cool the stirred solution to 10°C with ice-salt water, Add 10 mL of propylene oxide dropwise. By controlling the dropping rate to 1 drop / sec and maintaining the temperature at 0 °C. After reacting for 30 minutes, the volatile matter was extracted in time, and then 20 mL of pre-prepared epichlorohydrin was...
example 3
[0027] In the 100mL four-necked flask, drop into the chloropropane of 30g and the catalyst of 6.5g (phosphotungstopolyacid / TiO 2 ), then add 10mL hydrogen peroxide and 5g diamine hydrogen phosphate under stirring state, and react for 6 hours under the reflux of chloropropane; after the reaction is completed, the system is divided into oil and water phase suspensions after standing for 1-2 hours; the oil layer is obtained by separating the essence 23.36mL of epichlorohydrin for use. Then add 5g of diethylaluminum chloride and 50ml of anhydrous diethyl ether dried over molecular sieves to a three-necked flask equipped with a stirring bar, and stir for 10 minutes at room temperature, cool the stirred solution to 0°C with ice-salt water, and Add 10 mL of propylene oxide dropwise. By controlling the dropping rate to 1 drop / sec and maintaining the temperature at 20 °C. After reacting for 30 minutes, the volatile matter was extracted in time, and then 20 mL of pre-prepared epichlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com