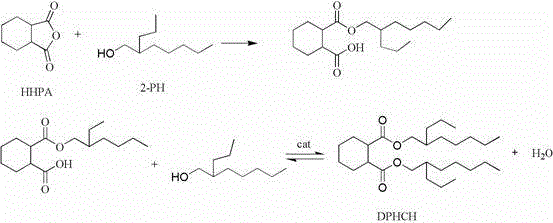
Preparation method of di(2-propylheptyl) cyclohexyl-1,2-diformate
A dicarboxylic acid di-ester plasticizer technology, which is applied in the preparation of carboxylic acid esters, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of product coloring, low purity, low product yield, poor reaction selectivity, etc. problems, to achieve the effect of basically colorless appearance, no three waste pollution, high plasticizing performance and mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
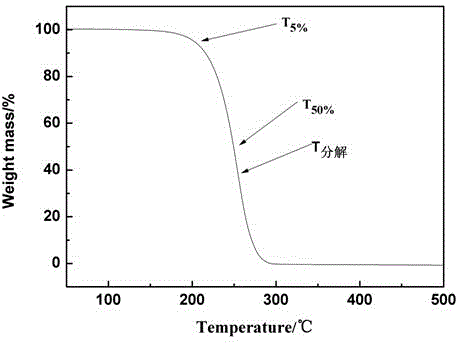
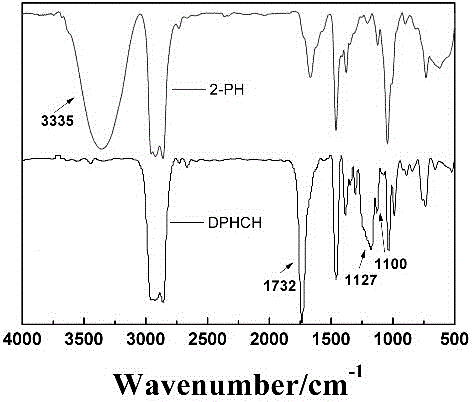
Image
Examples
Embodiment 1
[0020] Step 1 Esterification Reaction
[0021] Add 0.1mol (15.4g) of hexahydrophthalic anhydride and 2.8mol (44.3g) of 2-propylheptanol into a 250mL three-neck flask equipped with a magnetic stirrer, thermometer, reflux condenser, and water separator, and heat up to 100°C. Stir for 10 minutes until a transparent, uniform and stable liquid is formed. The measured acid value is 126.4mgKOH / g, then add 0.23g (1.49% of the anhydride content) catalyst tetrabutyl titanate into the three-necked flask, add 5mL of cyclohexane, and heat to The temperature is 200°C, heated to reflux and stirred, so that the water generated by the reaction is separated from the water separator, and the acid value is measured every 1 hour. The acid value is determined according to GB / T 1668-2008. After 4 hours of reaction, the acid value drops to 0.49mgKOH / g, the esterification rate is 99.62%, and the reaction is completed, and the product color is light yellow transparent liquid.
[0022] Purification of...
Embodiment 2
[0026] Step 1 Esterification Reaction
[0027] Add 0.1mol (15.4g) of hexahydrophthalic anhydride and 2.6mol (41.1g) of 2-propylheptanol into a 250mL three-neck flask equipped with a magnetic stirrer, thermometer, reflux condenser, and water separator, raise the temperature to 100°C, and magnetically Stir for 10 minutes until a transparent, uniform and stable liquid is formed. The measured acid value is 125.1mgKOH / g, then add 0.23g (1.49% of the anhydride content) catalyst tetrabutyl titanate into the three-necked flask, add 5mL of cyclohexane, and heat to The temperature is 200°C, heated to reflux and stirred, so that the water generated by the reaction is separated from the water separator, and the acid value is measured every 1 hour. The acid value is determined according to GB / T 1668-2008. After 5 hours of reaction, the acid value drops to 0.86mgKOH / g, the esterification rate is 99.31%, and the reaction is completed, and the product color is light yellow transparent liquid...
Embodiment 3
[0030] Step 1 Esterification Reaction
[0031] Add 0.1mol (15.4g) of hexahydrophthalic anhydride and 2.8mol (44.3g) of 2-propylheptanol into a 250mL three-neck flask equipped with a magnetic stirrer, thermometer, reflux condenser, and water separator, and heat up to 100°C. Stir for 10 minutes until a transparent, uniform and stable liquid is formed, and the measured acid value is 125.3 mgKOH / g, then add 0.15 g (1% of the anhydride content) of catalyst sodium bisulfate monohydrate into the three-necked flask, add 5 mL of cyclohexane, and heat to The temperature is 200°C, heated to reflux and stirred, so that the water generated by the reaction is separated from the water separator, and the acid value is measured every 1 hour. The acid value is determined according to GB / T 1668-2008. After 3 hours of reaction, the acid value drops to 0.40mgKOH / g, the esterification rate is 99.68%, and the reaction is completed, and the product color is a yellow transparent liquid.
[0032] Pur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com