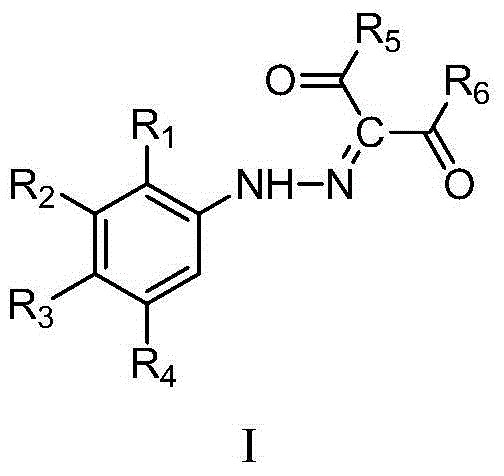
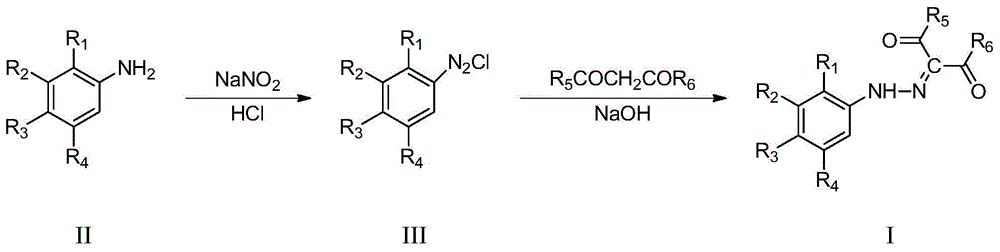
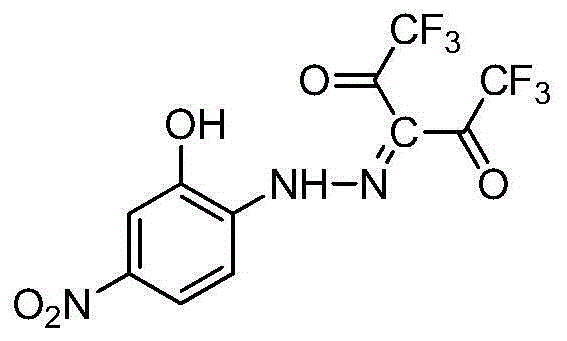
Phenylhydrazine fork-containing meta-dicarbonyl compound, preparation method and application thereof in inhibiting growth activity of candida albicans
A compound, dicarbonyl technology, applied in the field of inhibition of Candida albicans growth activity, can solve problems such as toxic and side effects, difficult to solve the problem of azole drug resistance, and achieve good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0069] The 6 compounds synthesized above were tested for enzymosome inhibition of Candida albicans fructose 1,6-bisphosphate aldolase. Fructose-1,6-bisphosphate aldolase can reversibly decompose fructose-1,6-bisphosphate into dihydroxyacetone phosphate and glyceraldehyde-3-phosphate. Under the action of triose phosphate isomerase (TIM), dihydroxyacetone phosphate can be converted into glyceraldehyde-3-phosphate. Finally, glyceraldehyde-3-phosphate is converted into glycerol-3-phosphate under the action of glyceraldehyde-3-phosphate dehydrogenase (GPDH), and at the same time, two molecules of NADH (nicotinamide adenine dinucleotide) are converted into NAD+. In the experiment, triose phosphate isomerase (TIM) and glyceraldehyde-3-phosphate dehydrogenase (GPDH) were used as coupling enzymes, and fructose 1,6-bisphosphate aldolase was determined by measuring the change of NADH absorbance at 340nm activity, and then reflect the inhibitory efficiency of the above-mentioned 6 kinds ...
Embodiment 3
[0071] 1. In vitro antifungal activity test:
[0072] The minimal inhibitory concentration (minimal inhibitory concentration) of the compounds to be screened against Candida albicans was detected by the Broth Microdilution method recommended by the American Clinical Laboratory Standards Institute (CLSI) CLSI-M27A3 and M38A2 documents. concentration, MIC), for antifungal activity screening. The inhibitory effects of compounds I-1 to I-6 on Candida albicans are shown in Table 1.
[0073] Table 1 The compound of general formula I is to Candida albicans FBA-II and to the inhibition of Candida albicans activity
[0074]
[0075] compound R 1 R 2 R 3 R 4 R 5 R 6 I 50 (μM) Mic 80 (μM) I-1 Oh H NO 2 H CF 3 CF 3 1.03±0.11 42.87
[0076] I-2 Oh SO 3 h H NO 2 CF 3 CF 3 8.35±1.47 35.30 I-3 Oh H H COOH CF 3 C 2 h 5 o 26.90±1.7 45.96 I-4 Oh H H NO 2 CF 3 C 2 h 5 o 3.17±0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com