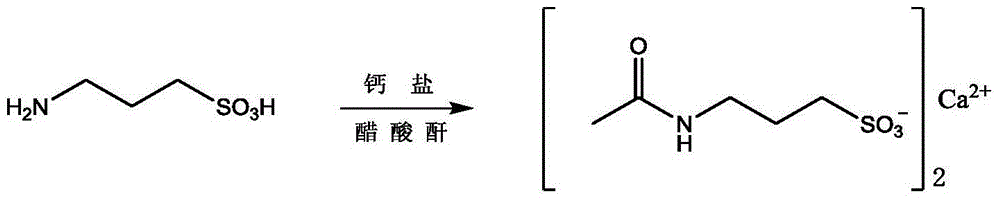
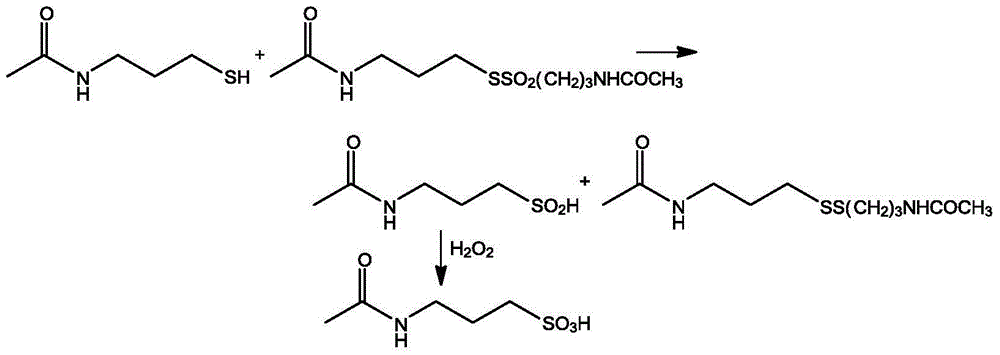
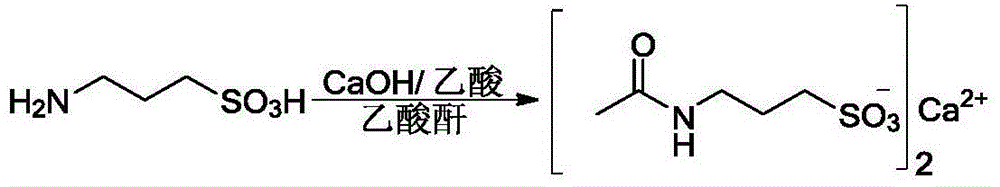
Method for preparing high-purity acetyl homotaurine
A technology of homotaurine and acetyl group is applied in the preparation field of synthesizing acetyl homotaurine, can solve the problems of difficult storage, harsh operation requirements, difficult quantification and the like, and achieves the effects of high yield and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Add 35 grams (252 mmol) of homotaurine into a 500 ml there-necked flask, add 300 ml of absolute ethanol, 58.50 grams of triethylamine (580 mmol) under stirring conditions, add 32.10 grams of acetic anhydride dropwise at room temperature, drop the mixture, and heat up Reflux reaction for 20-24 hours, add ice-water bath to cool down, dropwise add 45.74 g of acetyl chloride, dropwise at 20°C under temperature control, crystallize for 2 hours, filter, wash with 30ml of absolute ethanol, and dry under reduced pressure at 40°C to obtain 40.02 g of acetyl chloride For homotaurine, add 220ml of dichloromethane to reflux for 2 hours, filter, wash with 50ml of dichloromethane, and dry under reduced pressure at 40°C to obtain 22.5 g of acetyl homotaurine, HPLC: 99.35%, and homotaurine is less than 0.7%.
Embodiment 2
[0024] Example 2: Add 35 grams (252 mmol) of homotaurine into a 500 ml three-necked flask, add 300 ml of methylene chloride and 58.50 grams of triethylamine (580 mmol) under stirring, add 32.10 grams of acetic anhydride dropwise at room temperature, drop the mixture, and heat up Reflux reaction for 20-24 hours, add ice-water bath to cool down, add 55.34 g of acetyl chloride dropwise, add dropwise at 20°C under temperature control, crystallize for 2 hours, filter, wash with 30ml of absolute ethanol, and dry under reduced pressure at 40°C to obtain 45.02 g of acetyl chloride For homotaurine, add 220ml of dichloromethane to reflux for 2 hours, filter, wash with 50ml of dichloromethane, and dry under reduced pressure at 40°C to obtain 20.5 g of acetyl homotaurine, HPLC: 99.75%, and homotaurine is less than 0.7%.
Embodiment 3
[0025] Example 3: Add 35 grams (252 mmol) of homotaurine to a 500 ml three-necked flask, add 300 ml of absolute ethanol and 58.50 grams of triethylamine (580 mmol) under stirring, add 32.10 grams of acetic anhydride dropwise at room temperature, drop the mixture, and heat up Reflux reaction for 20-24 hours, add ice-water bath to cool down, add 67.72 grams of concentrated hydrochloric acid dropwise, add dropwise under temperature control at 20°C, crystallize for 2 hours, filter, wash with 30ml of absolute ethanol, and dry under reduced pressure at 40°C to obtain 44.02 grams of acetyl For homotaurine, add 220ml of anhydrous room temperature beating for 2 hours, filter, wash with 50ml of dichloromethane, and dry under reduced pressure at 40°C to obtain 18.5 g of acetyl homotaurine, HPLC: 99.05%, and homotaurine is less than 0.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com