Antitumor drug based on PGA-RB (Polyglutamic Acid-Rose Bengal) bound compound and preparation method and application of antitumor drug
A technology of tetrachlorotetraiodofluorescein and anti-tumor drugs, which is applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems such as no reports of tetrachlorotetraiodofluorescein sodium drug polymers, and achieve in vivo time The effect of long circulation, good water solubility, and long blood circulation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of antitumor drugs based on polyglutamic acid and tetrachlorotetraiodofluorescein bond
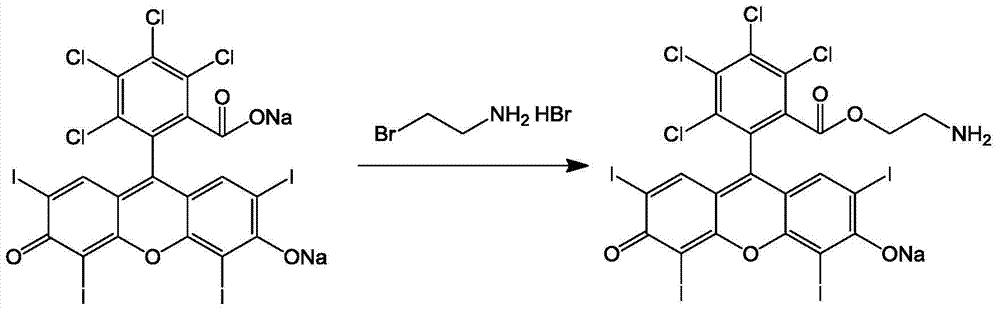
[0038] (1) Synthesis of tetrachlorotetraiodofluorescein-ethylamine (reaction principle sees figure 1 ):
[0039] ①Dissolve sodium tetrachlorotetraiodofluorescein (1.05g, 1.03mmol) and 2-bromoethylamine hydrobromide (0.45g, 2.20mmol) in 20mL of anhydrous DMF, and react in an oil bath at 80°C for 6h;
[0040] ② After the reaction is completed, evaporate the DMF to dryness under reduced pressure, add 50 mL of anhydrous ether, centrifuge, and wash three times;
[0041] ③Add 50mL water, centrifuge and wash 3 times;
[0042] ④Recrystallize in 10mL of methanol, filter and drain to obtain tetrachlorotetraiodofluorescein-ethylamine.
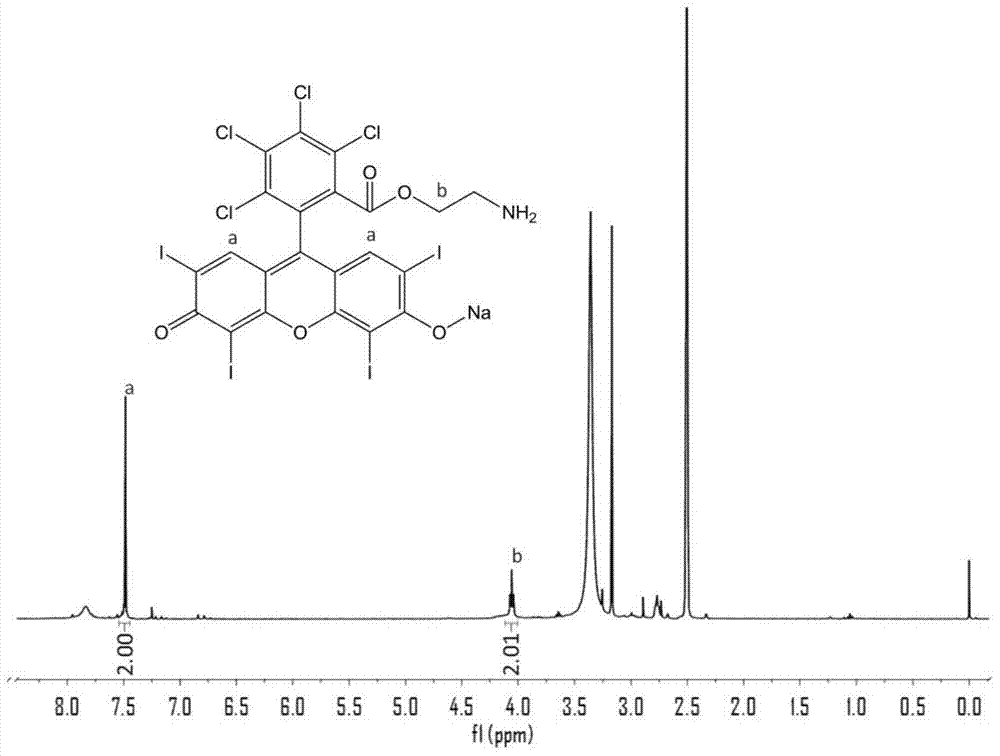
[0043] tetrachlorotetraiodofluorescein-ethylamine 1 H NMR (DMSO-d 6 ) characterization see figure 2 .
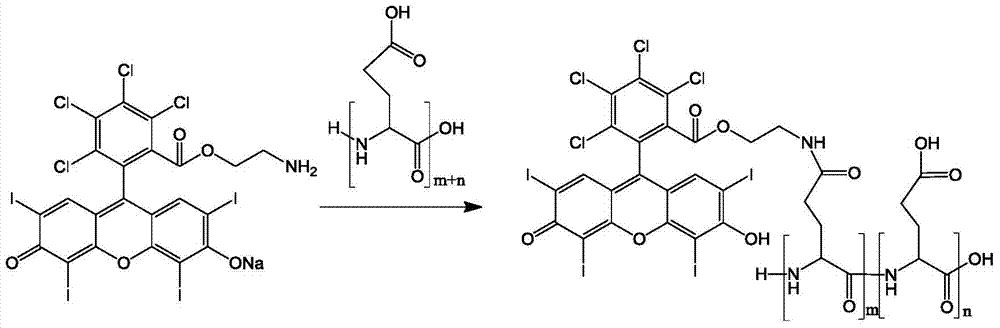
[0044](2) Synthesis of polyglutamic acid and tetrachlorotetraiodofluorescein bond (reaction principle see image 3 ):
[0045...
Embodiment 2
[0050] Example 2 Cytotoxicity measurement
[0051] Taking the polyglutamic acid and tetrachlorotetraiodofluorescein bond (hereinafter referred to as the bond) prepared in Example 1 as an example, the MTT (3-(4,5- Dimethylthiazole-2)-2,5-diphenyltetrazolium bromide) method was used to detect the cytotoxicity of the conjugate to cancer cell Bcap-37. Specific steps are as follows:
[0052] The Bcap-37 cells were respectively treated with the bond (final concentrations were 0.8mg / mL, 0.27mg / mL, 0.089mg / mL, 0.030mg / mL, 0.0099mg / mL, 0.0033mg / mL, 0.00037mg / mL, 0.00012mg / mL) (lighting experiment group and non-lighting experiment group), equal concentration of sodium tetrachlorotetraiodofluorescein (RB light group, RB non-light group), and equal concentration of linear polyglutamic acid (PGA group) After 3 hours, light was applied to the light experiment group and the RB light group for 1 hour (fluorescent lamp, with a wavelength of 500-600 nm) (the non-light experiment group, the RB...
Embodiment 3
[0054] Example 3 Blood Clearance Speed Test
[0055] Taking the polyglutamic acid and tetrachlorotetraiodofluorescein bonded compound prepared in Example 1 as an example, inject the same medicine (10 mg / kg) containing the equivalent of tetrachlorotetraiodofluorescein sodium to the tail vein of mice, and regularly take Blood, every 0.05ml of blood is precipitated with 0.95ml of acetonitrile, centrifuged to get the supernatant, and utilizes the fluorescence linear relationship (564nm excitation, 596nm emission) of the drug in an organic solvent to measure the drug concentration in the plasma. The results are as follows: Figure 8 shown. It can be seen that the in vivo circulation time of polyglutamic acid and tetrachlorotetraiodofluorescein-bonded nanoparticles prepared in Example 1 is longer than that of the small-molecule drug of tetrachlorotetraiodofluorescein sodium.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Lc50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com