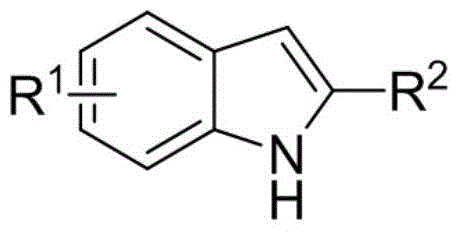
A kind of synthetic method of quinazolinone aromatic compound
A quinazolinone aromatic and synthesis method technology, applied in the direction of organic chemistry, can solve the problems of harsh reaction conditions, narrow substrate range, and long reaction time, and achieve short reaction time, wide substrate range, and green post-treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of 2-phenyl-3-phenethylquinazolin-4(3H)-one
[0022] Add 0.25mmol of 2-phenylindole, 0.5mmol of phenethylamine, 0.0125mmol of cuprous bromide, and 2.0mL of N-methylpyrrolidone into a 10mL reaction tube, place it in an oil bath at 80°C, and react under the protection of oxygen 20h. Stop the reaction and cool to room temperature. The reaction solution was diluted with dichloromethane, extracted three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 After drying, filtering, and separation by column chromatography, 77.5 mg of the target product was obtained, with a yield of 96%. The NMR characterization of this compound is as follows: 1 HNMR (400MHz, DMSO-d 6 ):δ8.24(d,J=8.0Hz,1H),7.94–7.80(m,1H),7.68(d,J=8.1Hz,1H),7.63–7.48(m,6H),7.25–7.12( m,3H),6.92–6.72(m,2H),4.1(t,J=7.8Hz,2H),2.83(t,J=7.8Hz,2H); 13 C NMR (100MHz, DMSO-d 6 )δ161.1, 155.9, 146.8, 137.8, 135.2, 134.5, 129.6, 128.5, 128.3 (overlapped), 127.9, 127.2, 127.0, 126...
Embodiment 2
[0024] Preparation of 2-phenyl-3-phenethylquinazolin-4(3H)-one
[0025] Add 0.25mmol of 2-phenylindole, 0.5mmol of phenethylamine, 0.0125mmol of cuprous bromide, and 2.0mL of N, N dimethylformamide into a 10mL reaction tube, place in an oil bath at 80°C, and oxygen Reaction under protection for 20h. Stop the reaction and cool to room temperature. The reaction solution was diluted with dichloromethane, extracted three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Dried, filtered, and separated by column chromatography to obtain 77.5 mg of the target product with a yield of 86%.
Embodiment 3
[0027] Preparation of 2-phenyl-3-phenethylquinazolin-4(3H)-one
[0028] Add 0.25mmol of 2-phenylindole, 0.5mmol of phenethylamine, 0.0125mmol of cuprous bromide, and 2.0mL of N, N dimethylacetamide into a 10mL reaction tube, place in an oil bath at 80°C, and oxygen Reaction under protection for 20h. Stop the reaction and cool to room temperature. The reaction solution was diluted with dichloromethane, extracted three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 After drying, filtering, and separation by column chromatography, 77.5 mg of the target product was obtained, with a yield of 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com