A tumor-targeted nano-drug delivery system modified by tandem penetrating peptides recognized by dual receptors
A penetrating peptide and tandem technology, which is applied in the field of tumor-targeted nano-drug delivery systems modified by tandem penetrating peptides recognized by dual receptors, can solve problems such as limiting the application of penetrating peptides.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
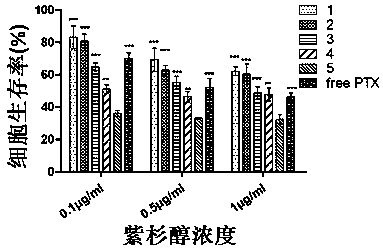
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 R8-dGR
[0033] (1) Take 1g of 2-CL-Trt Resin resin and place it in a solid-phase reactor, add 15ml of dichloromethane (DCM) to swell for 30min, and then drain the DCM;
[0034] (2) Weigh 0.9 g of arginine protected by fluorenylmethoxycarbonyl (Fmoc) at the N-terminal, add it to the swollen resin, add N,N-diisopropylethylamine (DIEA), dimethylamide ( DMF), DCM nitrogen bubbling reaction at room temperature for 2.5 hours, drain the reaction solution after the reaction, add 20ml of ethyl acetate to wash, then add 20% hexahydropyridine / DMF solution to remove the Fmoc protecting group, add ethyl acetate after removing the protecting group 20ml wash;
[0035] (3) Weigh 0.67 g of glycine protected by fluorenylmethoxycarbonyl (Fmoc) at the N-terminal, add it to the reactor, and then add benzotriazole-N,N,N',N'-tetramethyluronium hexafluoro Phosphate ester (HBTU), DIEA, react at room temperature for 2 hours with nitrogen gas bubbling, add 20ml ...
Embodiment 2
[0040] Example 2. DSPE-PEG 2000 - Preparation of R8-dGR
[0041] In a 25ml round bottom flask, add 10ml chloroform followed by DSPE-PEG 2000 -Mal 15.0mg (5.1umol), triethylamine 3ul (17.9mmol), then R8-dGR 13.1mg (7.7umol) was dissolved in 5ml of methanol and added to a round bottom flask. Press and spin dry to get the initial product, add chloroform to dissolve, filter, and spin dry under reduced pressure to get DSPE-PEG 2000 -R8-dGR 21.8 mg (92.0%), a colorless oily compound.
[0042] Based on the method of this embodiment, when PEG is selected from 200-50000, R X When selected from R2, R3, R4, R5, R6, R7, R8, R9, R10, R11, R12, when DGR is selected from DGR, dGR, DGr, dGr (lowercase letters represent D-type amino acids), Lipid is selected from DSPE , PE, DOPE, DPPE, DMPE, DEPE, each specific Lipid-PEG-R can be similarly prepared X -DGR.
Embodiment 3
[0043] Example 3. R X - Preparation of DGR (X=2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12) modified liposomes
[0044] With phospholipids, cholesterol, DSPE-PEG 2000 、DSPE-PEG 2000 -R X -DGR is used as material to prepare double receptor recognition tumor targeting liposome. Weigh 1.4mg of phospholipids, 0.4mg of cholesterol, DSPE-PEG 2000 0.3mg, DSPE-PEG 2000 -R X -DGR0.2mg, dissolved in an appropriate amount of chloroform, in a water bath at 37°C, remove the chloroform by rotary evaporation under reduced pressure to form a uniform lipid film, add 1ml of PBS buffer for hydration for 1 hour, and ultrasonically (10s, 10s, 15 times) with the probe. Then extrude through 400nm, 200nm and 100nm polycarbonate films in turn to obtain R X - DGR modified liposomes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com