A kind of preparation method of polyglycolide
A technology of polyglycolide and glycolide, which is applied in the field of aminoanilinolithium compounds and its preparation, can solve the problems of rapid development of polymer materials, aggravated pollution, difficult degradation of polymer materials, etc., and achieve catalyst structure Variety of effects with less metal residue and controllable molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
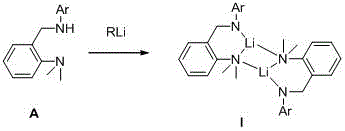
[0043] The structural formula of the ligand used is the above formula (A), where Ar is 4-methylphenyl, and the reaction process is as follows: under a nitrogen atmosphere, dissolve 0.51 g of the ligand in 10 mL of dry n-hexane, and add equimolar A certain amount of n-butyllithium was slowly raised to room temperature for 3 hours, filtered and washed with dry n-hexane, collected and dried to weigh 0.49g solid, yield 94%.
[0044] The NMR information of the obtained product is as follows. It can be seen from the NMR information that the lithium compound whose Ar is 4-methylphenyl was successfully synthesized.
[0045] 1 HNMR (400MHz, CDCl 3 ) δ 7.40(d, J =7.5Hz,2H,Ph- H ), 7.24–6.91 (m, 10H, Ph- H ),6.62(d, J =7.6Hz,2H,Ph- H ),4.40(s,4H,C H 2 N),2.72(s,12H,NC H 3 ),2.20(s,6H,C H 3 ).
[0046] 13 CNMR (100MHz, CDCl 3 ) δ152.45, 146.61, 134.27, 129.81, 128.23, 127.98, 126.66, 124.17, 116.34, 114.34, 48.86, 45.46, 20.40.
Embodiment 2
[0048] The structural formula of the ligand used is the above formula (A), where Ar is 2,6-dimethylphenyl, and the reaction process is as follows: under a nitrogen atmosphere, dissolve 0.47g of the ligand in 10mL of dry cyclohexane, and at 0°C Add 1.05 times the molar amount of n-butyllithium, slowly rise to room temperature and react for 6 hours, filter and wash the filter cake with dry n-hexane, collect and dry and weigh to obtain 0.46g solid, yield 95%.
[0049] The NMR information of the obtained product is as follows. It can be seen from the NMR information that the lithium compound whose Ar is 2,6-dimethylphenyl was successfully synthesized.
[0050] 1 HNMR (400MHz, CDCl 3 ) δ 7.42 (dd, J =6.2,1.3Hz,2H,Ph- H ),7.28-7.25(m,2H,Ph- H ),7.17(dd, J =7.0,1.0Hz,2H,Ph- H ),7.06(td, J =7.4,1.2Hz,2H),7.00(d, J =7.4Hz,4H),6.83(t, J =7.5Hz,2H),4.17(s,4H,NC H 2 Ph),2.73(s,12H,NC H 3 ),2.31(s,12H,PhC H 3 ).
[0051] 13 CNMR (100MHz, CDCl 3 ) δ 152.80, 146.54, 135...
Embodiment 3
[0053] The structural formula of the ligand used is the above formula (A), where Ar is 2,6-diethylphenyl, and the reaction process is as follows: under a nitrogen atmosphere, dissolve 0.34 g of the ligand in 5 mL of dry toluene, and add 1.1 times the molar amount of methyllithium was raised to room temperature and reacted for 1 hour, filtered and washed with dry n-hexane, collected, dried and weighed to obtain 0.32g, with a yield of 91%.
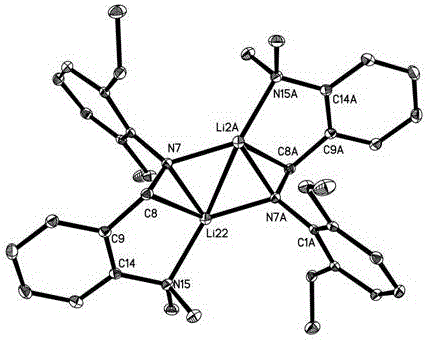
[0054] The crystal structure of the obtained product is shown in figure 1 As shown, the NMR information is as follows, it can be seen that the lithium compound whose Ar is 2,6-diethylphenyl was successfully synthesized.
[0055] 1 HNMR (400MHz, CDCl 3 ) δ 7.48–7.45 (m,2H,Ph- H ),7.29(dd, J =7.8,1.5Hz,2H,Ph- H ),7.19(dd, J =8.0,1.1Hz,2H,Ph- H ),7.13(d, J =7.9Hz,2H,Ph- H ),7.09(m,4H,Ph- H ),6.97(dd, J =8.1,6.9Hz,2H,Ph- H ),4.12(s,4H,NC H 2 Ph),2.73(s,12H,NC H 3 ),2.52(q, J =7.5Hz,8H,C H 2 CH 3 ),1.15(t, J =7.5Hz,12H,CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com