Preparation method for 5-(N, N-dibenzylamino)acetylsalicylamide
A technology of acetyl salicylamide and chloroacetyl salicylamide, which is applied to the preparation of carboxylic acid amides, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of high cost and low yield, and achieve low production cost, The effect of high yield and large operation pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
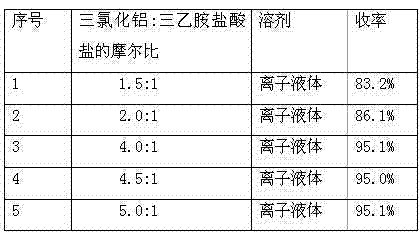
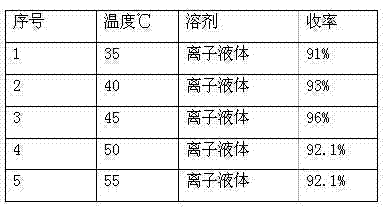
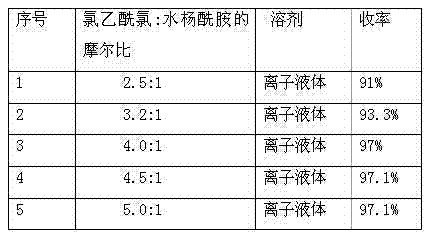
[0026] A simple and efficient method for preparing 5-(N,N-dibenzylamino)acetylsalicylamide, which uses salicylamide and chloroacetyl chloride as raw materials, and ionic liquid (triethylamine hydrochloride-aluminum trichloride) As a solvent and catalyst, react at 45°C to obtain 5-chloroacetylsalicylic amide, and the obtained 5-chloroacetylsalicylic amide reacts with dibenzylamine to obtain 5-(N,N-dibenzylamino)acetylsalicylic acid Amide, concrete steps are as follows:
[0027] Add salicylamide (10g, 0.073mol) and 50g of ionic liquid (triethylamine hydrochloride-aluminum chloride) into a three-necked flask, and after stirring evenly, add chloroacetyl chloride (20.43g, 0.18mol) dropwise at room temperature, After the dropwise addition, the oil bath controls the reaction temperature at 45°C for 2-3 hours, and monitors the reaction with TCL. After the reaction is completed, extract with ethyl acetate for 3-4 times, and spin dry the extracted ethyl acetate layer to obtain a white s...
Embodiment 2
[0030] A simple and efficient method for preparing 5-(N,N-dibenzylamino)acetylsalicylamide, which uses salicylamide and chloroacetyl chloride as raw materials, 1,2-dichloroethane as solvent and trichloro Aluminum catalyst, react at 45°C to obtain 5-chloroacetylsalicylicamide, and then react the obtained 5-chloroacetylsalicylicamide with dibenzylamine to obtain 5-(N,N-dibenzylamino)acetyl water Salicylic amide, concrete steps are as follows:
[0031] Add salicylamide (10g, 0.073mol) and 50g of 1,2-dichloroethane into a three-necked flask. After stirring evenly, add 35g of aluminum trichloride (the same quality as that contained in 50g of ionic liquid) ), and then add chloroacetyl chloride (20.43g, 0.18mol) dropwise at room temperature. After the dropwise addition, the oil bath controls the reaction temperature to 45°C for 2-3 hours. TCL monitors the reaction. After the reaction is completed, extract with ethyl acetate 3-4 times, the extracted ethyl acetate layer was spin-dried...
Embodiment 3
[0035] A simple and efficient method for preparing 5-(N,N-dibenzylamino)acetylsalicylamide, which uses salicylamide, chloroacetyl chloride as raw materials, nitrobenzene) as solvent and aluminum trichloride as catalyst , react at 45° C. to obtain 5-chloroacetylsalicylic amide, and then react the obtained 5-chloroacetylsalicylic amide with dibenzylamine to obtain 5-(N, N-dibenzylamino) acetylsalicylic amide, Specific steps are as follows:
[0036]Add salicylamide (10g, 0.073mol) and 50g of nitrobenzene into a three-necked flask. After stirring evenly, add 35g of aluminum trichloride (the same quality as that contained in 50g of ionic liquid), and then Chloroacetyl chloride (20.43g, 0.18mol) was added dropwise. After the dropwise addition, the oil bath controlled the reaction temperature to 45°C for 2-3 hours. TCL monitored the reaction. After the reaction was completed, extracted 3-4 times with ethyl acetate. The extracted ethyl acetate layer was spin-dried to obtain 9.34 g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com