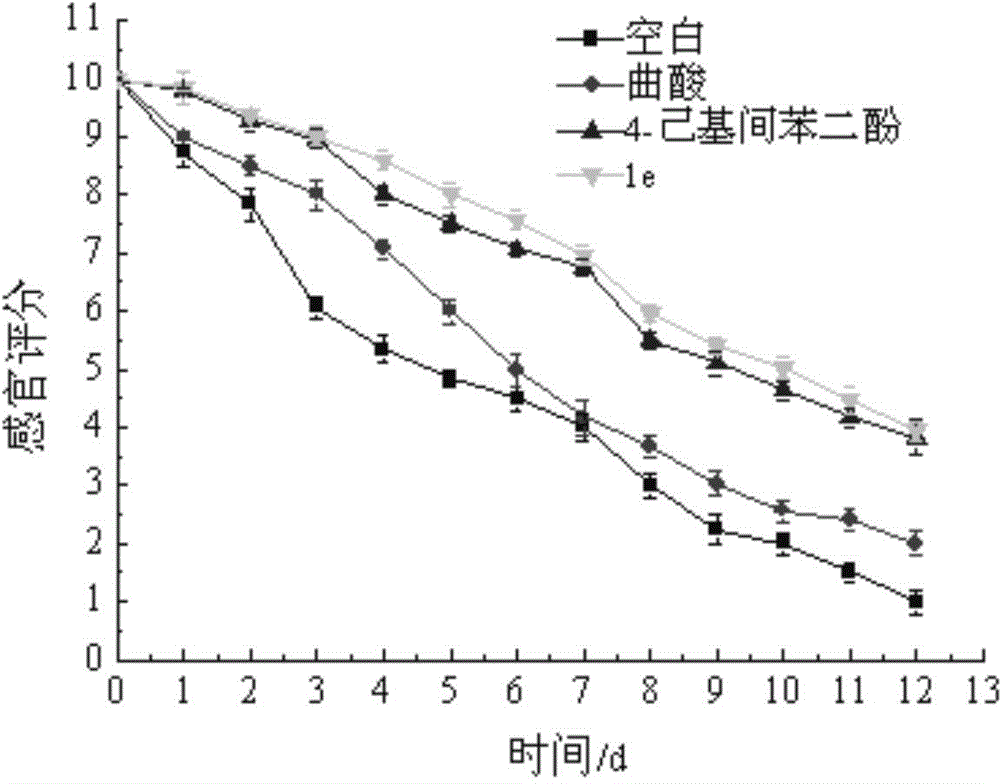
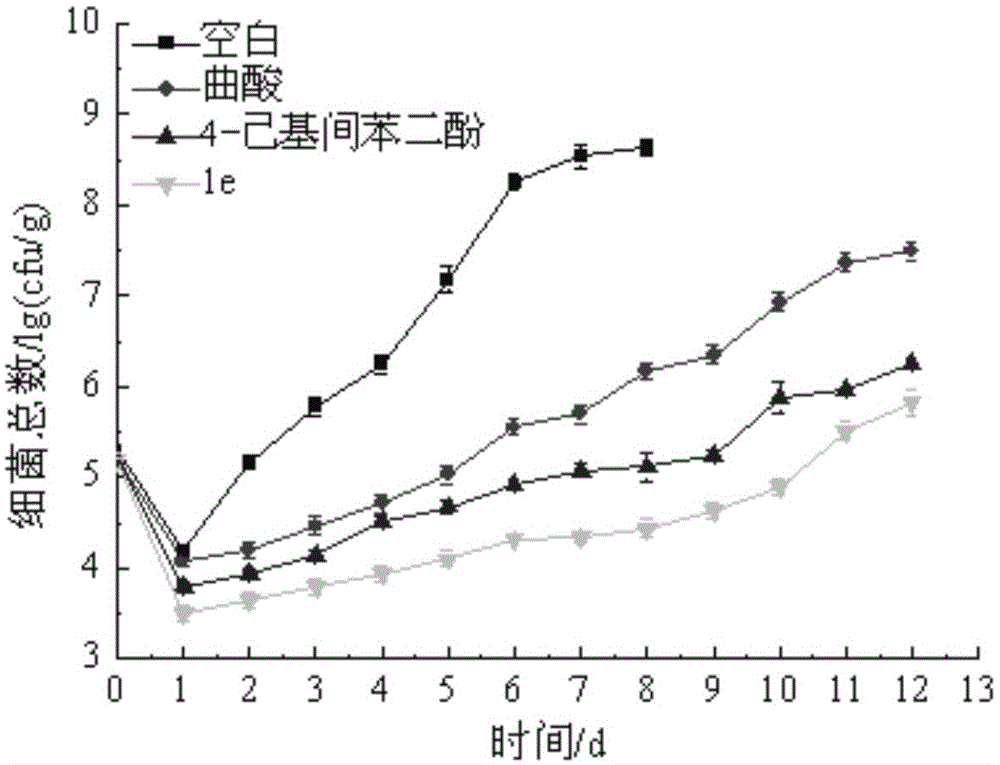
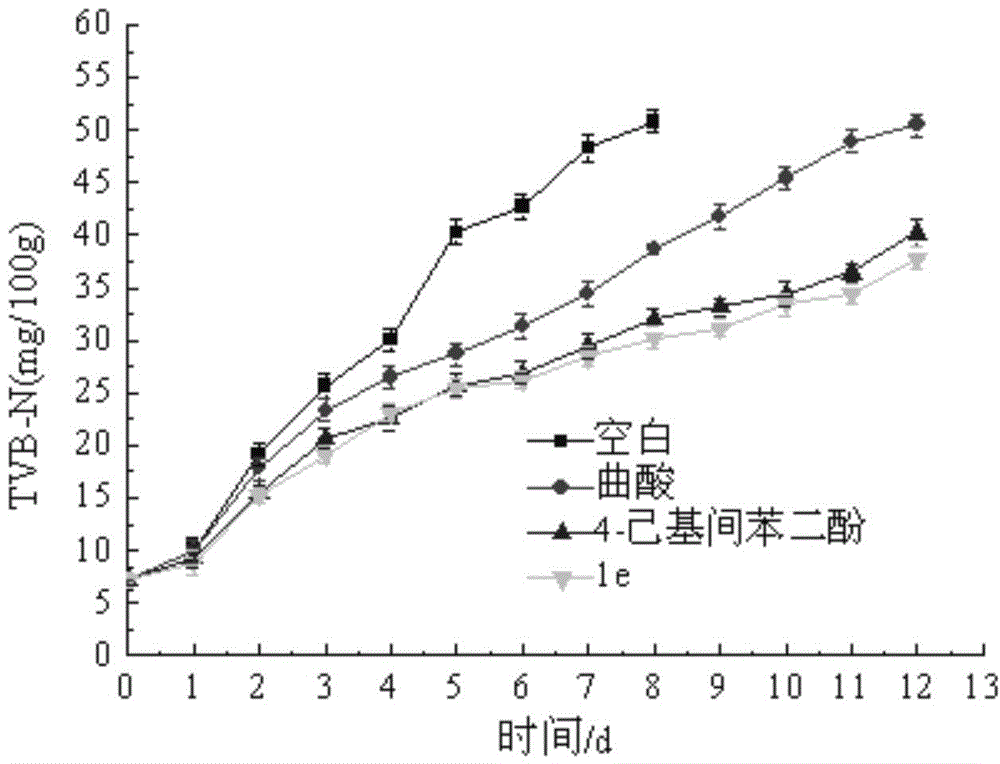
Hyroxy-pyridone derivative with multiple biological activities and usage thereof
A technology of hydroxypyridone and biological activity, which is applied in the direction of drug combination, cosmetic preparations, and chemical preservation of meat/fish, etc. It can solve the problems of unsatisfactory structural stability of kojic acid derivatives, achieve improved stability, Highly stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, the synthesis of compound 1:
[0030] The route to synthesize compound 1 from kojic acid is shown in Scheme 1.
[0031]
[0032] 5-Benzyloxy-2-hydroxymethyl-4H-pyran-4-one (4) (hereinafter referred to as compound 4) was synthesized according to the method reported in the literature (Design and synthesis of hydroxypyridinone-L-phenylalanine conjugates as potential tyrosinase inhibitors. Journal of Agricultural and Food Chemistry 2013, 61(27), 6597-6603).
[0033] Synthesis of A, 5-benzyloxy-2-hydroxymethylpyridin-4(1H)-one (5)
[0034] Take 30g of compound 4 and add 50mL of ethanol, then add 250mL of ammonia water (25-28%, wt%), reflux overnight (about 12 hours), and detect the reaction by TLC until the raw material point disappears. After the reaction was completed, cool to room temperature, the precipitate was separated out, and the precipitate was collected by filtration, then washed twice with a small amount of ether (50ml ether each time), and dri...
Embodiment 2
[0055] Embodiment 2, the synthesis of compound 2:
[0056] The route synthesis of compound 2 is shown in Scheme 2.
[0057]
[0058] Synthesis of A, 2-aminomethyl-5-benzyloxypyridin-4(1H)-one (7)
[0059]Dissolve 10 g (43.24 mmol) of compound 5 in 215 mL of thionyl chloride, and stir at room temperature for 18 h. The reaction solution was concentrated to obtain a pale yellow solid. Then add 100mL of methanol to dissolve the solid, add 200mL of ammonia water, stir at room temperature for 2h, then heat to 40°C for 8h, and cool to room temperature. The reaction solution was concentrated to dryness, and the obtained residue was separated and purified by silica gel column chromatography (methanol:dichloromethane=1:10 volume ratio), and the purification was specifically: the silica gel column contained 300g of silica gel; Methane=1:10 (volume ratio) as washing solution; collect R f =0.35 eluent, concentrated to obtain 6.91g (30.06mmol) product 7, namely 2-aminomethyl-5-benzyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com