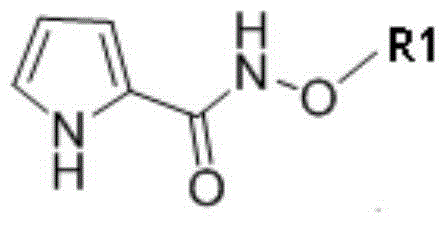
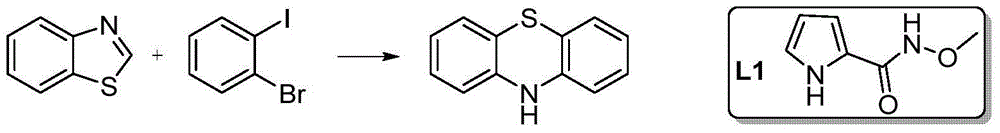
Novel method for synthesizing phenothiazine compounds
A technology of phenothiazines and compounds, which is applied in the field of synthesizing phenothiazines, can solve the problems of synthesizing phenothiazines that have not been reported, and achieve the effects of low solvent toxicity, simple operation and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the synthesis of phenothiazine
[0025]
[0026] 135mg (1mmol) benzothiazole, 566mg (2mmol) o-bromoiodobenzene, 25mg (0.1mmol) CuSO 4 ·5H 2 O, 14 mg (0.1 mmol) Ligand L1, 138 mg (1 mmol) K 2 CO 3 , 2g PEG-400, added to a 10mL reaction tube, sealed, and reacted at 50°C for 20h. After the reaction stopped, it was extracted with ethyl acetate, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure, separated and purified by silica gel column chromatography to obtain 160 mg of phenothiazine with a yield of 80%.
[0027] MS (EI + ):m / z:199(M + ,100),167(M-S,65); 1 H NMR (400MHz, DMSO-d 6 )δ8.61(s,1H,NH),6.99(td,J=7.6,1.4Hz,2H,ArH),6.94–6.88(m,2H,ArH),6.78–6.69(m,4H,ArH).
Embodiment 2
[0028] Embodiment 2: the synthesis of 2-chlorophenothiazine
[0029]
[0030]135 mg (1 mmol) benzothiazole, 158 mg (0.5 mmol) 2-bromo-4-chloro-1-iodobenzene, 16 mg (0.2 mmol) CuO, 2 mg (0.01 mmol) ligand L2, 28 mg (0.5 mmol) KOH, Add 2g of PEG-300 into a 10mL reaction tube, seal it, and react at 90°C for 3h. After the reaction stopped, it was extracted with ethyl acetate, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was distilled under reduced pressure, separated and purified by silica gel column chromatography to obtain 151 mg of 2-chlorophenothiazine, the yield 65%.
[0031] MS (EI + ):m / z:198(M-Cl,100),233(M + ,78),235(M + ,25),201(M-S,25). 1 H NMR (400MHz, DMSO-d 6 )δ8.77(brs,1H,NH),7.04–6.98(m,1H,ArH),6.96–6.88(m,2H,ArH),6.82–6.75(m,2H,ArH),6.73–6.66(m ,2H,ArH).
Embodiment 3
[0032] Embodiment 3: the synthesis of 2-fluorophenothiazine
[0033]
[0034] 135 mg (1 mmol) benzothiazole, 300 mg (1.0 mmol) 2-bromo-4-fluoro-1-iodobenzene, 6.4 mg (0.1 mmol) Cu, 21.6 mg (0.1 mmol) ligand L2, 978 mg (3 mmol) Cs 2 CO 3 , 2g PEG-100, added to a 10mL reaction tube, sealed, and reacted at 110°C for 14h. After the reaction stopped, extracted with ethyl acetate, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was distilled under reduced pressure, separated and purified by silica gel column chromatography to obtain 156 mg of 2-fluorophenothiazine, the yield 72%.
[0035] MS (EI + ):m / z:217(M + ,100),185(M-S,88). 1 H NMR (400MHz, DMSO-d 6 )δ8.81(s,1H,NH),7.01(td,J=7.7,1.5Hz,1H,ArH),6.97–6.89(m,2H,ArH),6.78(t,J=7.5Hz,1H, ArH), 6.70(d, J=7.9Hz, 1H, ArH), 6.59(td, J=8.5, 2.7Hz, 1H, ArH), 6.53(dd, J=10.5, 2.6Hz, 1H, ArH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com