Ledipasvir and sofosbuvir compound coating tablet preparation and preparation method thereof
A technology of coated tablets and preparations, applied in the direction of sugar-coated pills, pill delivery, antiviral agents, etc., to achieve stable blood drug concentration and improve convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
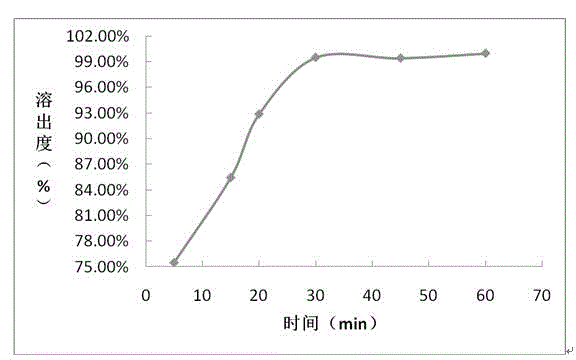
Embodiment 1
[0043] prescription:
[0044] Composition Prescription amount (mg / tablet)
[0045] Reddy Pawee 90
[0046] Sofosbuvir 400
[0047] Croscarmellose Sodium 96
[0048] Microcrystalline Cellulose 502
[0050] According to above-mentioned prescription quantity, preparation process is as follows:
[0051] (1) Material pretreatment: sieve ledipasvir, sofosbuvir, croscarmellose sodium, microcrystalline cellulose, and magnesium stearate, and set aside;
[0052] (2) Mixing: Weigh the prescription amount of the active drug ledipasvir, sofosbuvir, croscarmellose sodium, microcrystalline cellulose, and magnesium stearate and mix evenly;
[0053] (3) Place the mixed powder in (2) in a dry granulator for granulation;
[0054] (4), total mixing: uniformly mix the dry granules prepared in (3) with the prescribed amount of croscarmellose sodium and magnesium stearate;
[0055] (5) Press plain tablets: place the granules prepared in (4) in a high-speed rot...
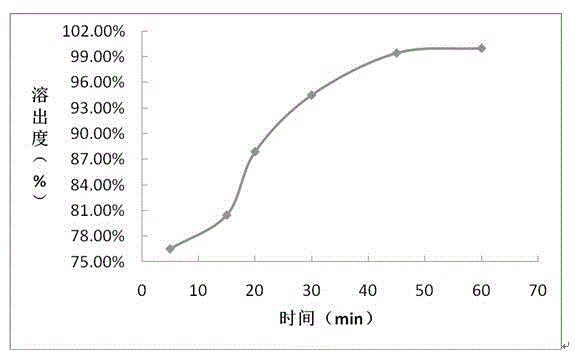
Embodiment 2
[0058] prescription:
[0059] Composition Prescription amount (mg / tablet)
[0060] Reddy Pawee 90
[0061] Sofosbuvir 400
[0062] Hypromellose 96
[0063] Microcrystalline Cellulose 502
[0065] According to above-mentioned prescription quantity, preparation process is as follows:
[0066] (1) Material pretreatment: sieve ledipasvir, sofosbuvir, hypromellose, microcrystalline cellulose, and magnesium stearate, and set aside;
[0067] (2) Mixing: Weigh the prescribed amount of active drug ledipasvir, sofosbuvir, hypromellose, microcrystalline cellulose, and magnesium stearate and mix evenly;
[0068] (3) Place the mixed powder in (2) in a dry granulator for granulation;
[0069] (4), total mixing: uniformly mix the dry granules prepared in (3) with the prescribed amount of hypromellose and magnesium stearate;
[0070] (5) Press plain tablets: place the granules prepared in (4) in a high-speed rotary tablet press;
[0071] (6) Film coati...
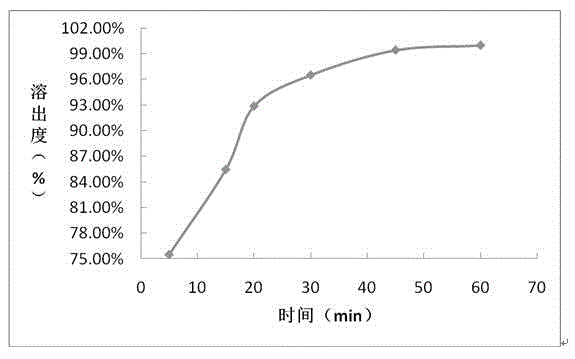
Embodiment 3
[0073] prescription:
[0074] Composition Prescription amount (mg / tablet)
[0075] Reddy Pawee 90
[0076] Sofosbuvir 400
[0077] Croscarmellose Sodium 96
[0078] Mannitol 502
[0080] According to above-mentioned prescription quantity, preparation process is as follows:
[0081] (1) Material pretreatment: sieve ledipasvir, sofosbuvir, croscarmellose sodium, mannitol, and magnesium stearate, and set aside;
[0082] (2) Mixing: Weigh the prescription amount of the active drug ledipasvir, sofosbuvir, croscarmellose sodium, mannitol, and magnesium stearate and mix evenly;
[0083] (3) Place the mixed powder in (2) in a dry granulator for granulation;
[0084] (4), total mixing: uniformly mix the dry granules prepared in (3) with the prescribed amount of croscarmellose sodium and magnesium stearate;
[0085] (5) Press plain tablets: place the granules prepared in (4) in a high-speed rotary tablet press;
[0086] (6) Film coating: film-coa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com