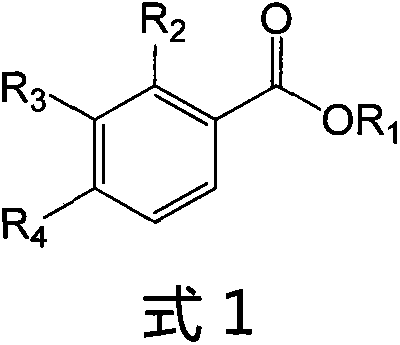
Ligustrazine substituted benzoic acid derivative (LQC-A) with neuroprotective activity and application of ligustrazine substituted benzoic acid derivative
A technology of benzoic acid derivatives, applied in ligustrazine derivatives and its preparation, preparation method and its application in neuroprotection, ligustrazine substitution of benzoic acid derivatives field, can solve clinical application limitations, traditional Chinese medicine compound Unclear ingredients, insufficient understanding of mechanism of action, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
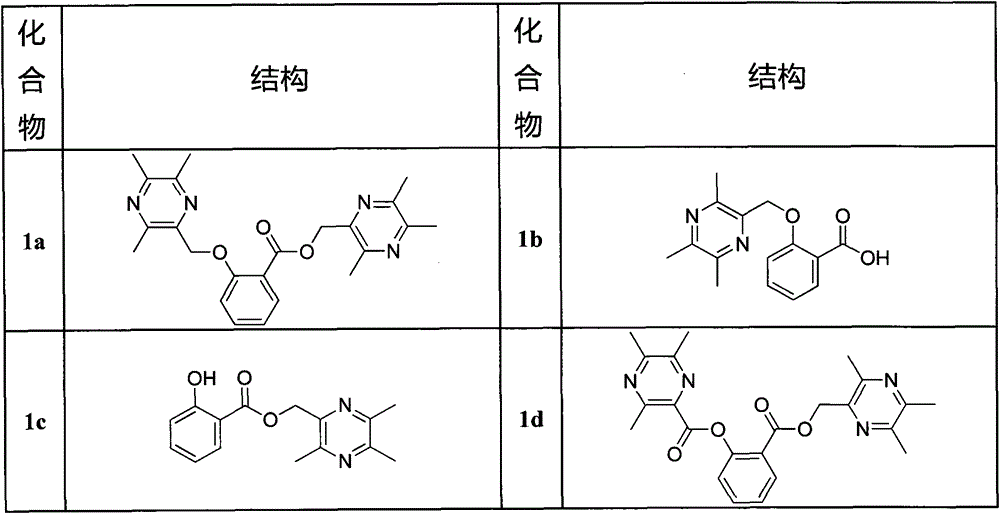
[0050] (3,5,6-trimethylpyrazin-2-yl)methyl
[0051] 2-[(3,5,6-trimethylpyrazin-2-yl)methoxy]benzoate (1a)
[0052] Weigh 10.14mmol of 2-bromomethyl-3,5,6-trimethylpyrazine and 5.07mmol of salicylic acid into a 50ml round bottom flask, add 30ml of DMF, and after the mixture dissolves, add 5mmol of potassium carbonate, Stir at 85°C for 2 hours, TLC monitors that the reaction raw materials basically disappear, stop the reaction, add a large amount of saturated NaCl solution to the reaction solution to disperse, extract twice with 300ml ethyl acetate, and wash the ethyl acetate layer with anhydrous NaCl 2 SO 4 Dry, concentrate under reduced pressure, add 4ml of chloroform to the residue to dissolve, add 3.8g of silica gel, evaporate to dryness under reduced pressure and mix the sample. The eluent is petroleum ether: acetone = 10:1, and 1.258g of white solid is obtained. M.P.: 76.2-76.9°C, yield 61.1%.
[0053] Structure Identification: 1 H-NMR (CDCl 3 )(ppm): 7.84(m, 1H, Ar-H...
Embodiment 2
[0055] 2-[(3,5,6-trimethylpyrazin-2-yl)methoxy]benzoic acid (1b).
[0056] Weigh 0.8g (1.97mmol) of 1a solid into a 100ml reaction flask, add 20ml of absolute ethanol, stir at 60°C until the solid is completely dissolved, add dropwise 8ml of 20% KOH solution, and the reaction time is about 30min. After the reaction is over, add 50ml of saturated NaCl solution into the reaction bottle, then add 4mol / L HCl dropwise to the reaction solution to adjust the pH to 3-4, and let stand until no white precipitates are precipitated. The reaction solution was suction filtered to obtain a precipitate, washed with distilled water until neutral, and dried to obtain 0.456 g of a white solid. M.P.: 172.2-172.9°C, yield 85.1%.
[0057] 1 H-NMR (CDCl 3 )(ppm): 8.10(d, 1H, J=7.5Hz, Ar-H), 7.53(t, 1H, J=8.0Hz, Ar-H), 7.16(d, 1H, J=8.5Hz, Ar- H), 7.12(t, 1H, J=7.5Hz, Ar-H), 5.40(s, 2H, -CH 2 ), 2.53 (brs, 6H, -CH 3 ), 2.52(s, 3H, -CH 3 ). 13 C-NMR (CDCl 3 )(ppm): 166.2, 157.0, 151.6, 149.1,...
Embodiment 3
[0059] (3,5,6-trimethylpyrazin-2-yl)methyl2-hydroxybenzoate (1c).
[0060] Weigh 10.87mmol of 2-bromomethyl-3,5,6-trimethylpyrazine and 10.87mmol of salicylic acid into a 50ml round bottom flask, add 25ml of DMF, and after the mixture is dissolved, add 10.0mmol of bicarbonate Sodium, stirred at room temperature for 12 hours, TLC monitoring reaction raw materials disappeared, stop the reaction, the reaction solution was dispersed by adding a large amount of saturated NaCl solution, 400ml ethyl acetate was extracted twice, the ethyl acetate layer was washed with anhydrous NaCl 2 SO 4 Dry, concentrate under reduced pressure, add 5ml of chloroform to the residue to dissolve, add 4.4g of silica gel, evaporate to dryness under reduced pressure and mix the sample. The eluent is petroleum ether: acetone = 12:1, and 1.892g of white solid is obtained. M.P.: 84.4-85.1°C, yield 64.0%.
[0061] 1 H-NMR (CDCl 3 )(ppm): 10.66(s, lH, -OH), 7.85(dd, J=8, 1.5Hz, 1H, Ar-H), 7.47(td, J=8, 1.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com