Preparation method and application of repaglinide
A reaction and post-reaction technology, applied in the field of drug synthesis, can solve problems such as unsuitable for large-scale industrial production, unfavorable for large-scale industrial production, harsh reaction conditions, etc., and achieve the effects of low cost, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
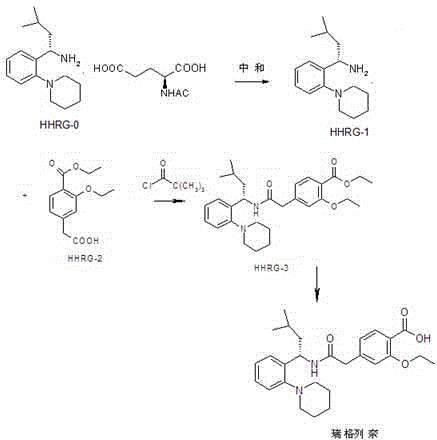
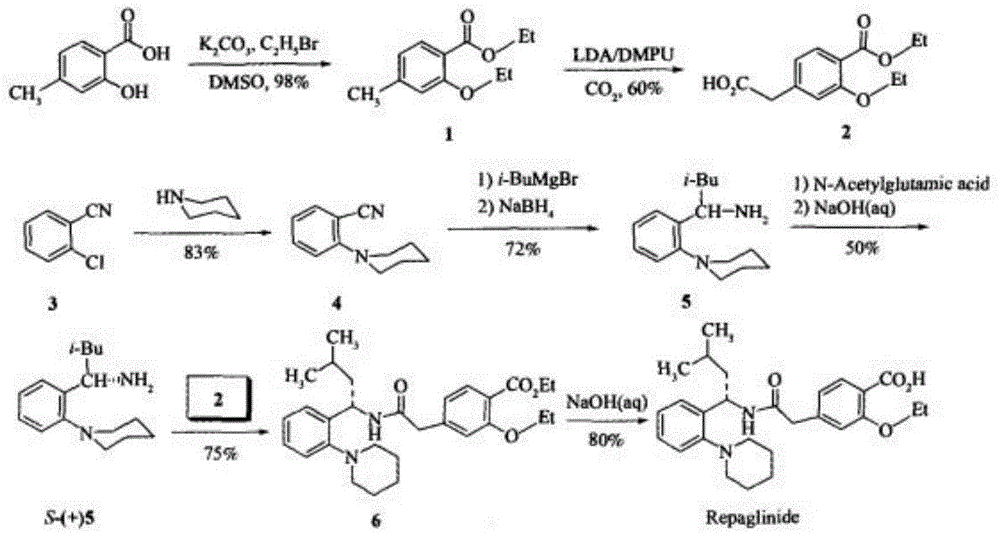
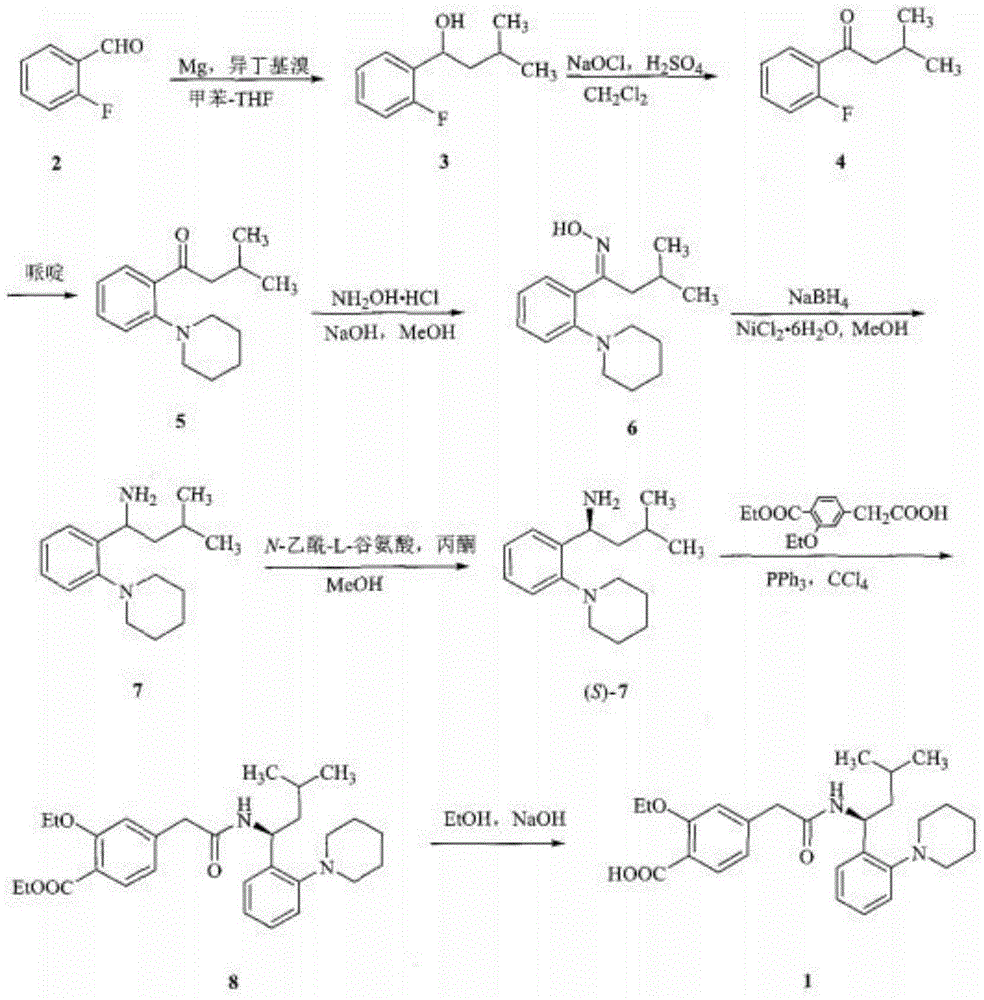
[0057] figure 1 The schematic flow chart of the preparation method of repaglinide provided by the embodiment of the present invention, combined with figure 1 , the embodiment of the present invention provides a kind of preparation method of repaglinide, the small test comprises the following steps:
[0058] Step 1: Preparation of compound B (HHRG-1)
[0059]
[0060] Add 250ml of water to a 1L three-necked round-bottomed flask, add 22g of compound A (regigamine-N-acetyl-L-glutamic acid) under stirring conditions, stir to form a suspension, add 20ml of ammonia water, stir until completely dissolved, and then Stir for 1 hour, add 300ml of dichloromethane and stir for 30 minutes, pour into a 1L separating funnel and let stand to separate layers, then extract the water layer with 200ml of dichloromethane, combine the organic layers, add anhydrous sodium sulfate to dry, filter, and the filtrate Concentrate under reduced pressure at 40°C to obtain 12g of light yellow oily liqui...
Embodiment 2
[0074] figure 1 The schematic flow chart of the preparation method of repaglinide provided by the embodiment of the present invention, combined with figure 1 , the embodiment of the present invention provides a kind of preparation method of repaglinide, pilot test comprises the following steps:
[0075] Step 1: Preparation of HHRG-1
[0076]
[0077] Add 2500ml of water to a 10L reaction kettle, add 220g of Regigamine-N-acetyl-L-glutamic acid under stirring conditions, stir to form a suspension, add 200ml of ammonia water, stir until completely dissolved, stir for another hour, add 3000ml Dichloromethane was stirred for 30 minutes, poured into a 10L separatory funnel and allowed to stand for stratification, the aqueous layer was extracted with 2000ml of dichloromethane, the organic layers were combined, dried with anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the The concentration temperature was 40°C, and about 121 g of HHR...
Embodiment 3
[0088] For further illustrating the beneficial effect of the present invention, the embodiment of the present invention has studied by different experimental conditions:
[0089] 1) The preparation conditions of HHRG-1;
[0090] 2) the impact of the acylation reaction temperature on the compound shown in formula D;
[0091] 3) The impact of hydrolysis time on the hydrolysis of the compound shown in formula D, including the following steps;
[0092] First, do the following comparison test:
[0093] Step 1: Preparation of HHRG-1
[0094]
[0095]
[0096] Add 250ml of water to a 1L three-neck round-bottom flask, add 22g of Regigamine-N-acetyl-L-glutamic acid under stirring conditions, stir to form a suspension, add 20ml of ammonia water, stir until completely dissolved, add 300ml of dichloromethane Stir for 30 minutes, pour into a 1L separatory funnel and let stand to separate layers, then extract the water layer with 200ml of dichloromethane, combine the organic layers...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com