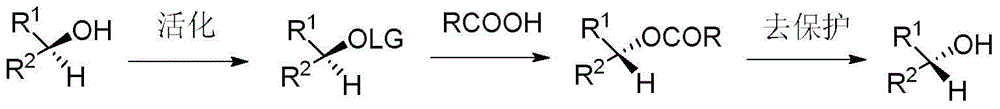
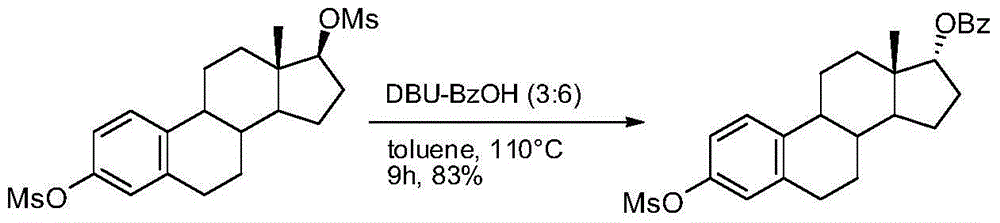
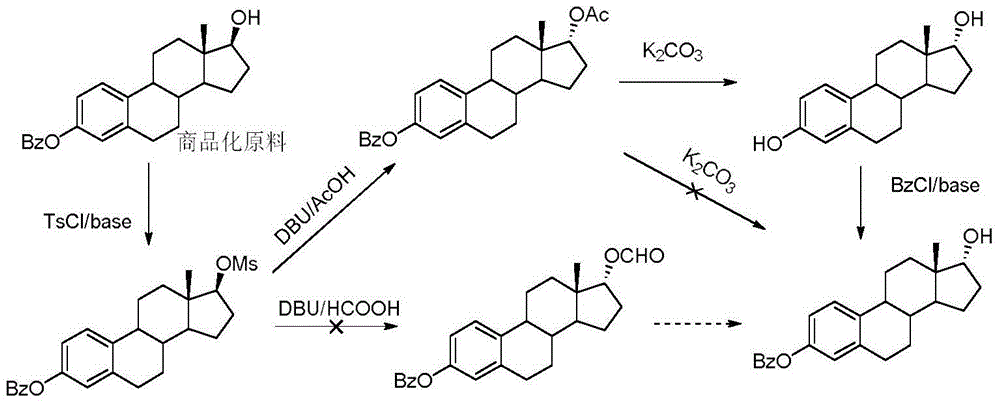
Synthesis method of 17-alpha-hydroxy steroid compounds
A technology of hydroxysteroids and synthetic methods, applied in the direction of steroids, chemical instruments and methods, organic chemistry, etc., can solve problems such as unrealized methods, avoid protection/deprotection process, increase applicable surface, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of Example 1 Fluorosulfonate 2
[0044]
[0045] The commercial compound 1 (947mg, 2.52mmol) was dissolved in 20mL of toluene, cooled in an ice bath, and DBU (0.90mL, 6.0mmol) and C 4 f 9 SO 2 F (0.54mL, 3.0mmol), the reaction was completed in about 10 minutes, poured into a short silica gel column, and washed with petroleum ether: ethyl acetate: dichloromethane = 10:1:1 to obtain about 1.58 g Product 2, yield 96%.
[0046] m.p.110℃
[0047] [α] D 25 =+7.33(c 1.0, CHCl 3 )
[0048] 1 H NMR (400MHz, CDCl3 )δ8.20(d, J=7.9Hz, 2H), 7.63(t, J=7.0Hz, 1H), 7.51(t, J=7.6Hz, 2H), 7.33(d, J=8.4Hz, 1H) ,6.99(d,J=8.5Hz,1H),6.94(s,1H),4.89(t,J=8.4Hz,1H),0.95(s,3H).
[0049] 19 F NMR (376MHz, CDCl 3 )δ-80.71(t,J=9.7Hz,3F),-111.26(dd,J=27.0,18.4Hz,2F),-121.26(d,J=9.0Hz,2F),-125.86–-126.09(m ,2F).
[0050] 13 C NMR (101MHz, CDCl 3 )δ165.4, 148.8, 138.0, 137.2, 133.5, 130.1, 129.6, 128.5, 126.5, 121.7, 118.9, 97.7, 48.6, 43.8, 43.7, 38.2, 35.9, 29.4, 27.8, 2...
Embodiment 21
[0051] Example 2 Synthesis of 17-α-hydroxyacetate 3
[0052]
[0053] Intermediate 2 (240mg, 0.36mmol) was dissolved in 3mL of toluene, acetic acid (83μL, 1.46mmol) and DBU (218μL, 1.46mmol) were added, and the reaction was completed after 0.5 hours. 3 Aqueous solution, saturated NH 4 Cl aqueous solution and saturated brine washing, anhydrous Na 2 SO 4 After drying, concentration and column chromatography. 3122 mg of the product was obtained with a yield of 80%.
[0054] m.p.148–149°C
[0055] [α] D 25 =+32.76(c 1.0, CHCl 3 )
[0056] 1 H NMR (400MHz, CDCl 3 )δ8.20(d, J=7.5Hz, 2H), 7.62(d, J=7.4Hz, 1H), 7.51(t, J=7.7Hz, 2H), 7.34(d, J=8.5Hz, 1H) ,6.98(d,J=8.4Hz,1H),6.94(s,1H),4.89(d,J=6.2Hz,1H),2.07(s,3H),0.80(s,3H).
[0057] 13 C NMR (101MHz, CDCl 3 )δ170.7, 165.4, 148.7, 138.3, 138.0, 133.4, 130.1, 129.7, 128.5, 126.5, 121.6, 118.7, 82.0, 49.1, 44.9, 43.8, 38.6, 31.8, 30.1, 29.6, 267.8, 33.2.0, 2 .
Embodiment 31
[0058] Example 3 Synthesis of 17-alpha-hydroxyformic acid ester 4
[0059]
[0060] The starting material (240 mg, 0.36 mmol) was dissolved in 3 mL of tetrahydrofuran, formic acid (55 μL, 1.46 mmol) and DBU (218 μL, 1.46 mmol) were added, and the reaction was completed after 0.5 hours. 3 Aqueous solution, saturated NH 4 Cl aqueous solution and saturated brine washing, anhydrous Na 2 SO 4 After drying, concentration and column chromatography. 3113 mg of the product was obtained with a yield of 78%.
[0061] [α] D 25 =+48.12(c 1.0, CHCl 3 ).
[0062] 1 H NMR (400MHz, CDCl 3 )δ8.20(d, J=7.5Hz, 2H), 7.62(d, J=7.4Hz, 1H), 7.51(t, J=7.7Hz, 2H), 7.34(d, J=8.5Hz, 1H) ,6.98(d,J=8.4Hz,1H),6.94(s,1H),4.89(d,J=6.2Hz,1H),2.07(s,3H),0.80(s,3H).
[0063] 13 C NMR (101MHz, CDCl 3 )δ165.4, 160.8, 148.7, 138.2, 137.8, 133.4, 130.1, 129.7, 128.5, 126.5, 121.6, 118.7, 81.9, 49.0, 44.9, 43.7, 38.6, 31.9, 30.1, 29.6, 27.8, 25.9, 2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com