Probiotic bacteria
A technology of probiotics and strains, applied in the field of probiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0187] Example 1: Materials and methods
[0188] bacterial growth
[0189] Probiotics were tested in Wilkins-Dahlgren (Wilkins-Dahlgren) at 37°C in Mark 3 (Mark 3) anaerobic workstation (Whitley Scientific, Shipley, UK) (Don Whitley Scientific, Shipley, UK). Chalgren) broth (WCB) (tryptone) was routinely grown to stationary phase. Staphylococcus aureus was grown aerobically at 37°C in nutrient broth (tryptone). Adjust the culture density spectrophotometrically to contain the desired number of bacteria. For experiments using keratinocytes, bacteria were washed twice in 0.85% NaCl solution, centrifuged and reconstituted in keratinocytes. Selective agar was used in some experiments. Mannitol salts agar-MSA (tryptone) or Man-Rogosa-Sharpe agar (MRS) for Staphylococcus aureus or Lactobacillus respectively. For experiments using heat-killed bacteria, L. reuteri was centrifuged and resuspended in 0.4% glucose and heat-inactivated by placing in a hot water bath at 85 °C for 4...
Embodiment 2
[0205] Staphylococcus aureus induces cell death in primary human keratinocytes
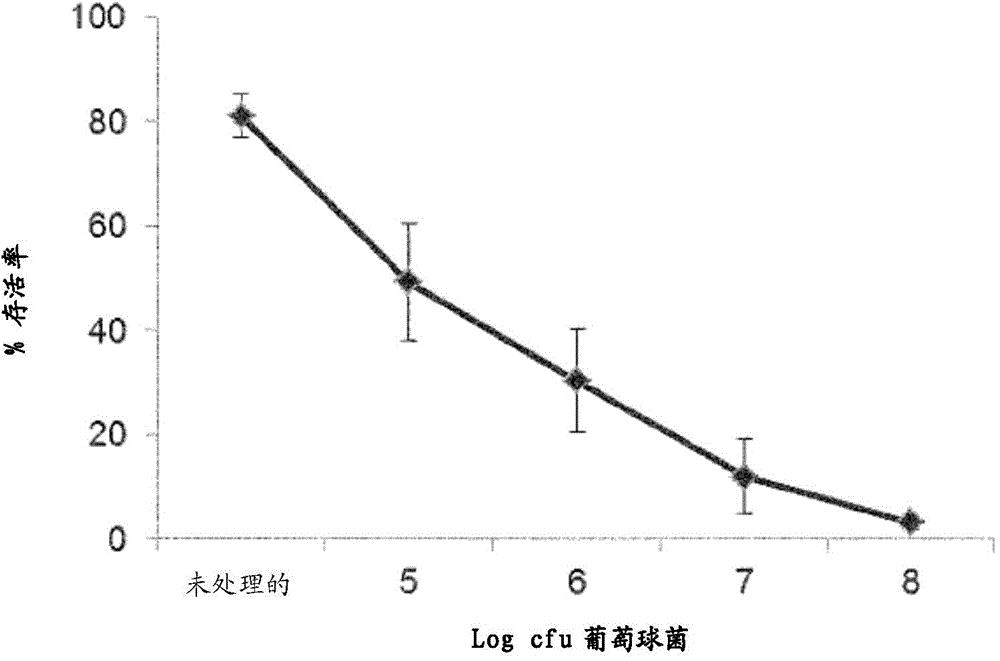
[0206] We initially demonstrated the effect of S. aureus on keratinocyte survival by performing a trypan blue exclusion test by incubating NHEK with S. aureus at different doses. The concentration of Staphylococcus aureus was 10 5 cfu / mL, only 49.4% of keratinocytes were alive after 24 hours of infection ( figure 1 ). The number of surviving keratinocytes was reduced in a dose-dependent manner, so that the concentration of Staphylococcus aureus at 10 8 At cfu / mL, only 3.3% of keratinocytes were viable ( figure 1 ).
[0207] about 10 6 This microorganism is likely to be found, for example, in physiologically relevant concentrations in skin wound infections (7). At this concentration, 30.5% of keratinocytes were viable 24 hours after infection. This concentration was used for the adhesion test.
[0208] Lactobacillus reuteri protects keratinocytes from Staphylococcus aureus-induced cell de...
Embodiment 3
[0234] As mentioned above, we demonstrated that L. reuteri is able to partially protect primary human keratinocytes from skin pathogens such as S. aureus. In this study, we have assessed the effect of a second probiotic, Lactobacillus rhamnosus GG as well as Lactobacillus rhamnosus GG, as well as Lactobacillus and bacterium Composition of lysates. Lactobacillus rhamnosus GG was isolated from human feces (see US Patent 4839281 and US Patent 5032399) and deposited under number ATCC 53103.
[0235] Lactobacillus reuteri protects keratinocytes from Staphylococcus aureus-induced cell death
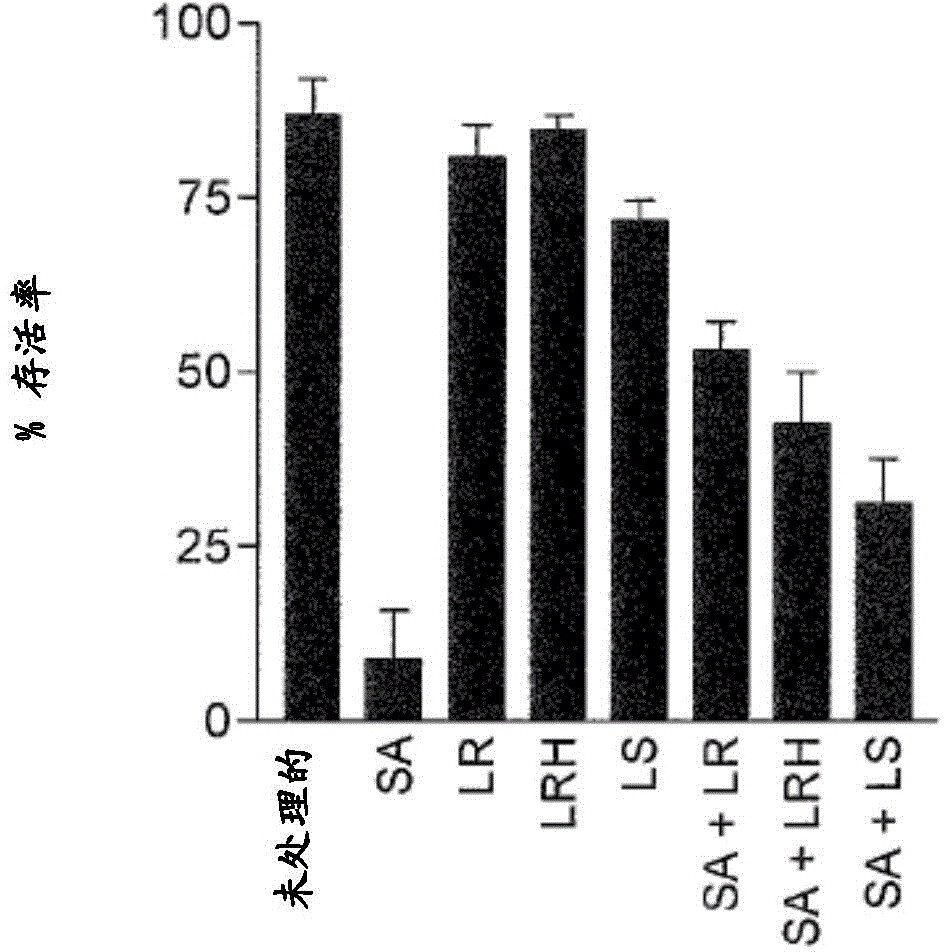
[0236] In the presence or absence of L. rhamnosus GG, L. reuteri alone or in combination (all at 10 8 / mL), normal human primary keratinocytes (KC) were exposed to Staphylococcus aureus (10 6 / mL). The survival of KCs was measured after 24 h using the trypan blue exclusion assay.
[0237]When KCs were treated with only S. aureus, only 39% of KCs were still alive 24 hours after infection (p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com