Scutellarin aglycone crystal forms and preparation method thereof
A technology of scutellarin aglycone and crystal form, which is applied in the field of medicine and chemical industry, and can solve the problems of complex components, poor oral absorption of scutellarin, and low oral utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] Example 1 Preparation of scutellarin aglycone crystal form A
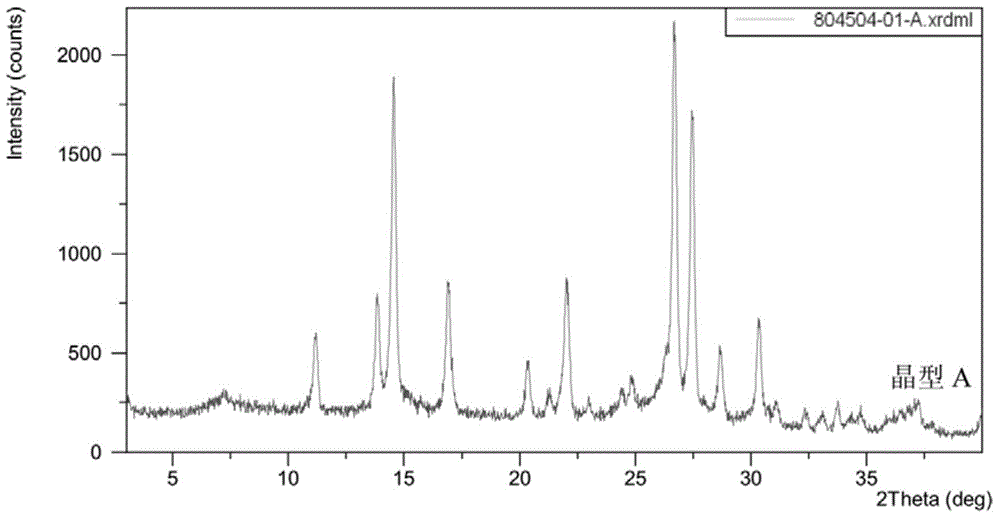
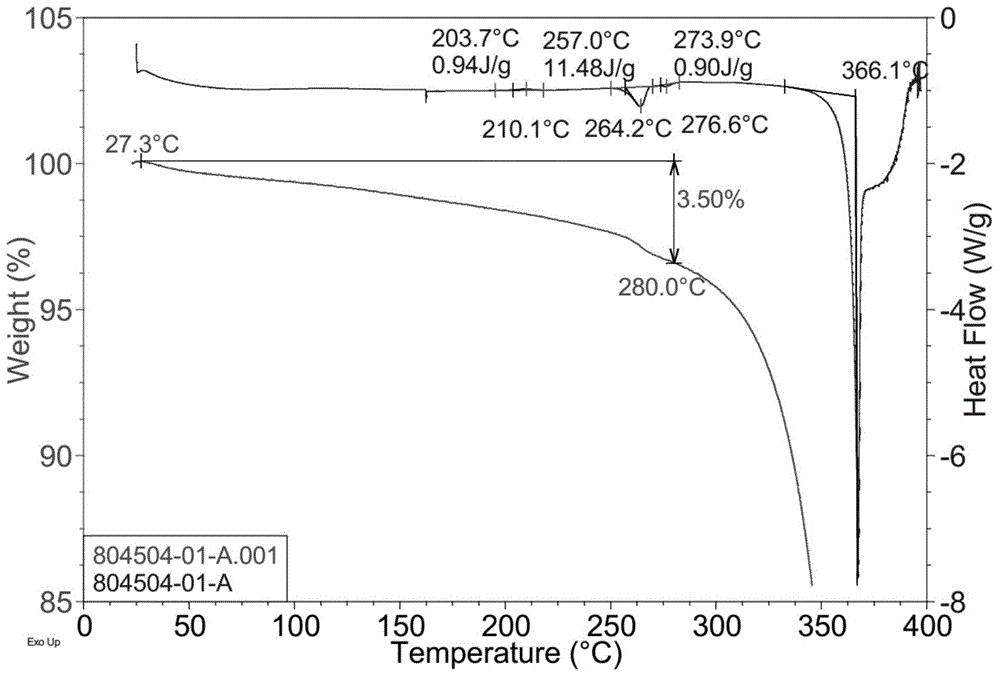
[0192]Take by weighing 100 grams of scutellarin (purchased from Yunnan Botanical Pharmaceutical Co., Ltd.), put it into a 5000ml round bottom flask, add 4000ml of propylene glycol or ethylene glycol, stir and heat to reflux, heat preservation and reflux to completely dissolve scutellarin; 20ml of 5% hydrochloric acid solution was slowly added dropwise to the solution; the reflux was continued for 6-16 hours, and the hydrolysis reaction was completed. After the solution is cooled, a large amount of precipitates are precipitated, filtered, and the filter cake is washed with propylene glycol or ethylene glycol and water respectively, dried and pulverized to obtain scutellarin aglycone crystal form A with a content (as determined by HPLC) of not less than 96%.
[0193] The typical XRPD figure of the crystal form A prepared according to this method is shown in figure 1 , the peak information of its spectrum is s...
Embodiment 2
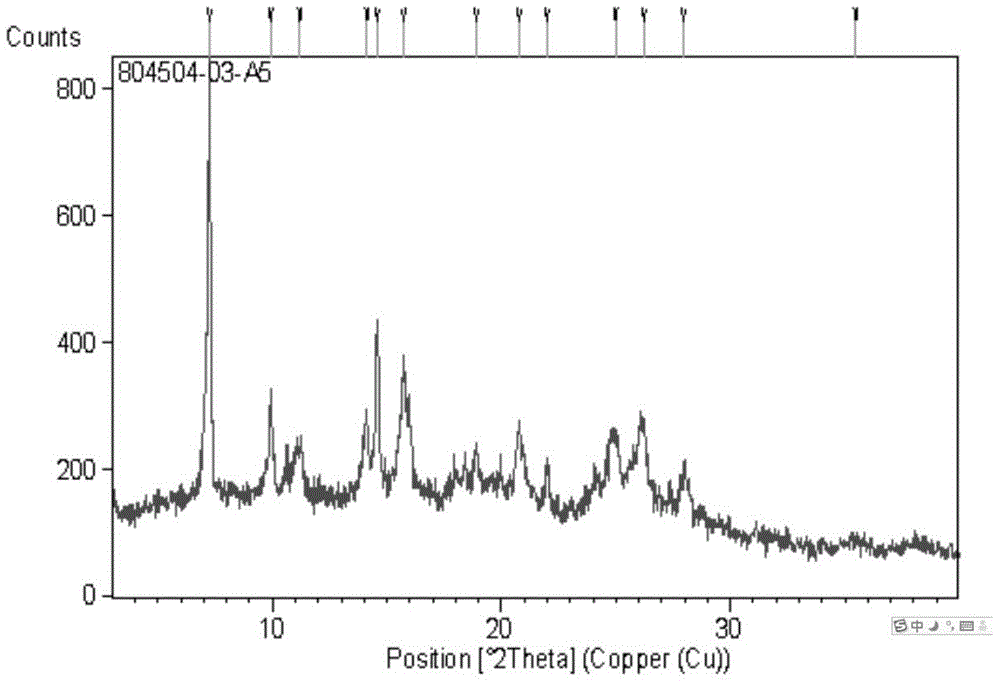
[0265] Example 2 Preparation of scutellarin aglycon crystal form B
[0266] Weigh about 10 mg of scutellarin aglycone crystal form A into a 3 mL glass bottle, add 0.5 to 1.25 mL of pyridine / water (volume ratio 3:1) or pyridine / acetonitrile (volume ratio 3:1) or pyridine / ethyl acetate ester (volume ratio 3:1), to ensure complete dissolution of the sample to obtain a clear solution. The obtained solution was slowly evaporated at room temperature, and the crystal form B was obtained after the solvent was evaporated.
[0267] Method 1: Use pyridine / water (volume ratio 3:1) as a solvent to obtain Form B, and its spectrum peak information is shown in Table 21.
[0268] Table 21 XRPD peak information of scutellarin aglycone crystal form B
[0269]
[0270] Method 2: Use pyridine / acetonitrile (volume ratio 3:1) as a solvent to obtain Form B, and its spectrum peak information is shown in Table 22.
[0271] Table 22 XRPD peak information of scutellarin aglycone crystal form B
[...
Embodiment 3
[0280] Example 3 Preparation of scutellarin aglycon crystal form C
[0281] Method 1: Weigh about 15 mg of scutellarin aglycone crystal form A into a 3 mL glass bottle, add 0.2 mL of pyridine to completely dissolve the solid, then slowly add 0.2-2 mL of heptane to the pyridine solution dropwise, if there is precipitation, put A solid sample was isolated by precipitation. If there is no precipitation, it evaporates rapidly at room temperature to obtain Form C, and its spectrum peak information is shown in Table 25.
[0282] Table 25 XRPD peak information of scutellarin aglycone crystal form C
[0283]
[0284]
[0285] Method 2: Weigh about 15 mg of scutellarin aglycone crystal form A into a 3 mL glass bottle, add 0.2 mL of pyridine to completely dissolve the solid. Slowly add the above clear solution dropwise to 5°C heptane, if there is precipitation, separate the precipitation to obtain a solid sample. If there is no precipitation, conduct a rapid volatilization test...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com