Method for improving expression level of trehalose synthase gene by molecular chaperone co-expression
A gene expression level, trehalose synthase technology, applied in the direction of transferase, the use of vectors to introduce foreign genetic material, recombinant DNA technology, etc., can solve the problems of molecular chaperones to improve the solubility of enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
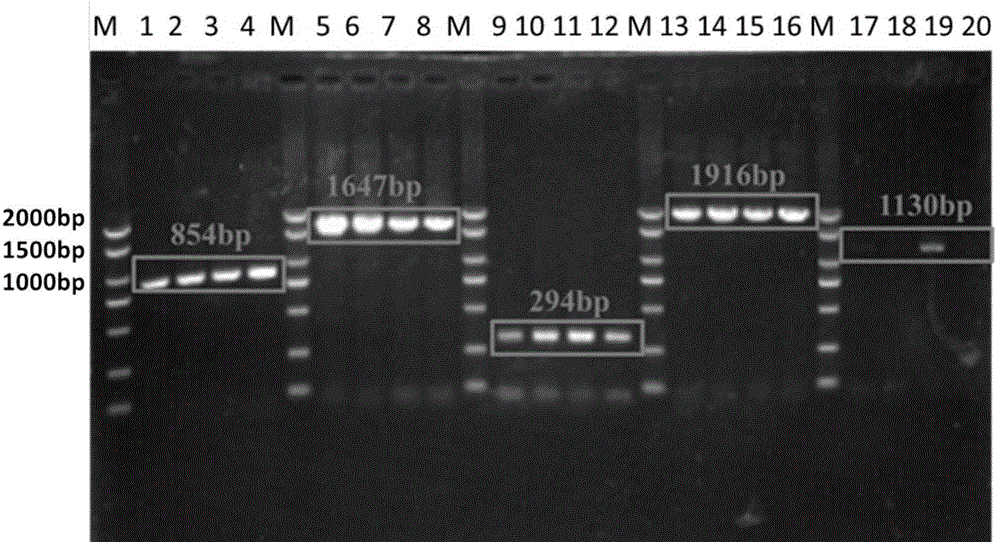
[0038] Example 1 Cloning of the thermophilic bacteria trehalose synthase gene (tttreS) coding region and its expression in Escherichia coli
[0039] 1. Primer design and PCR reaction
[0040] The genome of the thermophilic bacterium (Thermus thermophilus) HB27 was provided by Yan Yajun, a teacher from the University of Georgia, USA. This strain can be purchased from the China General Microorganism Culture Collection Center.
[0041] A pair of upstream and downstream primers were designed according to the sequence of the coding region of trehalose synthase derived from known thermophilic bacteria. The upstream was designed from the start codon, and the downstream primer was designed to the stop codon. The designed primers were:
[0042] Upstream primer 22tttreSNcoF: 5'-GGGAAA CCATGG GTGGACCCCCTCTGGTACAAG-3' (the underline is the NcoI restriction site),
[0043] Downstream primer 22tttreSSalR: 5'-GGGAAAGTCGAC GGCTTTTCCGGCCTTGGC-3' (the underline is the SalI restriction site); ...
Embodiment 2
[0086] Example 2 Cloning of molecular chaperone sigma32, GroeL, GroeS, DnaK and DnaJ gene coding regions and pCS27-sigma32, pCS27-GroeL-GroeS, pCS27-DnaK-DnaJ, pCS27-sigma32-GroeL-GroeS, pCS27-sigma32-DnaK- Construction of DnaJ, pCS27-GroeL-GroeS-DnaK-DnaJ and pCS27-sigma32-GroeL-GroeS-DnaK-DnaJ recombinant plasmids
[0087] 1. Extraction of the total genome of Escherichia coli BL21 (DE3)
[0088] Escherichia coli BL21 (DE3) preserved in the laboratory was inoculated into 4 mL of liquid LB medium, and cultured with shaking at 220 rpm for about 16 hours. The collected bacterial solution was added to a 2 mL centrifuge tube, centrifuged at 12000 rpm for 1 min at room temperature, and the supernatant was discarded for genome extraction. Add 180μL TE Buffer, resuspend the bacteria, then add 20μL 50mg / ml lysozyme solution, place in a water bath at 30°C for 10min, centrifuge at room temperature, 12000rpm for 5min, remove the supernatant, add 200μL Buffer BTL, oscillate to resuspend ...
Embodiment 3
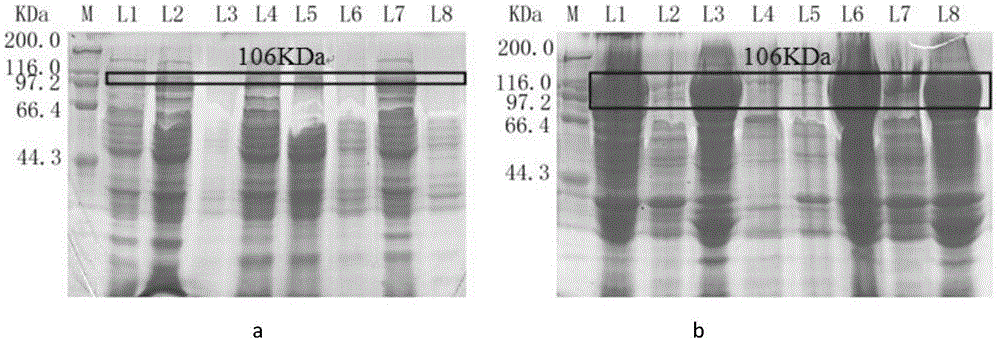
[0143] Example 3 Effect of co-expression of molecular chaperones on the production of trehalose synthase
[0144] 1. Construction of recombinant Escherichia coli containing trehalose synthase gene and molecular chaperone gene
[0145] Extract the pCS27-sigma32 constructed in Step 5 of Example 2, pCS27-GroeL-GroeS, pCS27-DnaK-DnaJ, pCS27-sigma32-GroeL-GroeS, pCS27-sigma32-DnaK-DnaJ, pCS27-GroeL-GroeS-DnaK-DnaJ and pCS27-sigma32-GroeL-GroeS-DnaK-DnaJ, and these seven plasmids containing molecular chaperones and plasmids containing trehalose synthase were co-heat-shock transformed into E. coli competent cells, mixed well, and the subsequent methods were the same as in the examples 1.
[0146] 2. High expression of trehalose synthase in recombinant Escherichia coli
[0147] Spread the above transformants on a solid plate (containing 50 μg / mL kanamycin sulfate and 100 μg / mL ampicillin), and culture at 37° C. overnight until colonies grow on the plate. Single colonies from the ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com