Preparation method for phenyl heptadecane
A technology of phenylheptadecane and phenyloctadecanoic acid, which is applied in the field of production of β-mannanase, can solve the problems of high energy consumption, high temperature, and poor safety, and achieve low energy consumption, low price, and safety sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
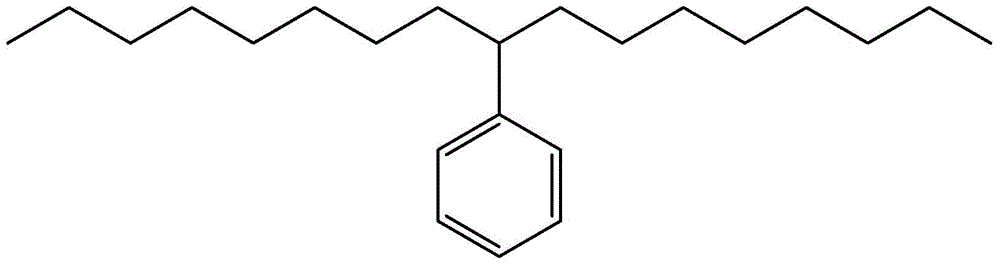
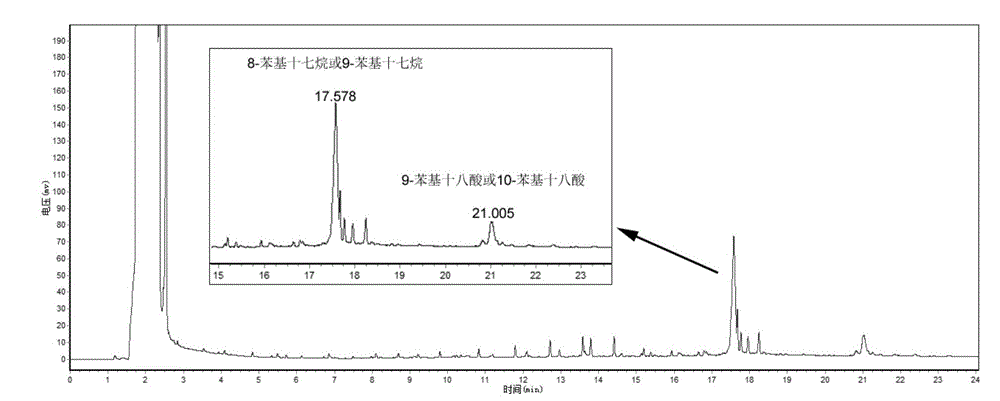
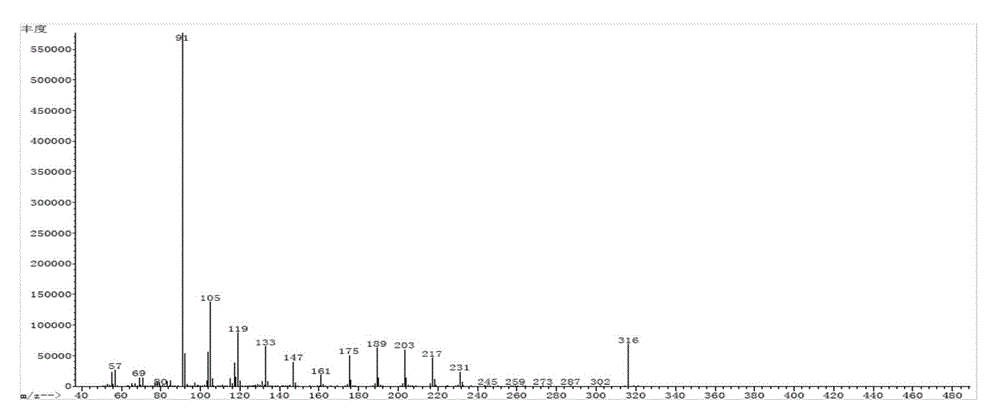
[0024] Take by weighing 9-phenyl octadecanoic acid or 10-phenyl octadecanoic acid 20g, catalyst 1.0g (mass ratio 20:1), join in the reactor, pass into N in the reactor 2 Gas for 20 minutes and seal the autoclave. Set the reaction temperature to 170°C, the reaction time to 6h, and the stirring speed to 300r / min. After the reaction, the obtained product was suction-filtered under reduced pressure, and after the catalyst was removed, 8-phenylheptadecane or 9-phenylheptadecane was obtained with a yield of 61.85%. The molecular structure of 9-phenylheptadecane is shown in figure 1 shown.
Embodiment 2
[0026] Take by weighing 9-phenyl octadecanoic acid or 10-phenyl octadecanoic acid 20g, catalyst 1.0g (mass ratio 20:1), join in the reactor, pass into N in the reactor 2 Gas for 20 minutes and seal the autoclave. Set the reaction temperature to 180°C, the reaction time to 6h, and the stirring speed to 300r / min. After the reaction, the obtained product was suction-filtered under reduced pressure, and the catalyst was removed to obtain 8-phenylheptadecane or 9-phenylheptadecane with a yield of 93.47%.
Embodiment 3
[0028] Take by weighing 9-phenyl octadecanoic acid or 10-phenyl octadecanoic acid 20g, catalyst 1.0g (mass ratio 20:1), join in the reactor, pass into N in the reactor 2 Gas for 20 minutes and seal the autoclave. Set the reaction temperature to 190°C, the reaction time to 6h, and the stirring speed to 300r / min. After the reaction, the obtained product was suction-filtered under reduced pressure, and the catalyst was removed to obtain 8-phenylheptadecane or 9-phenylheptadecane with a yield of 67.51%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com