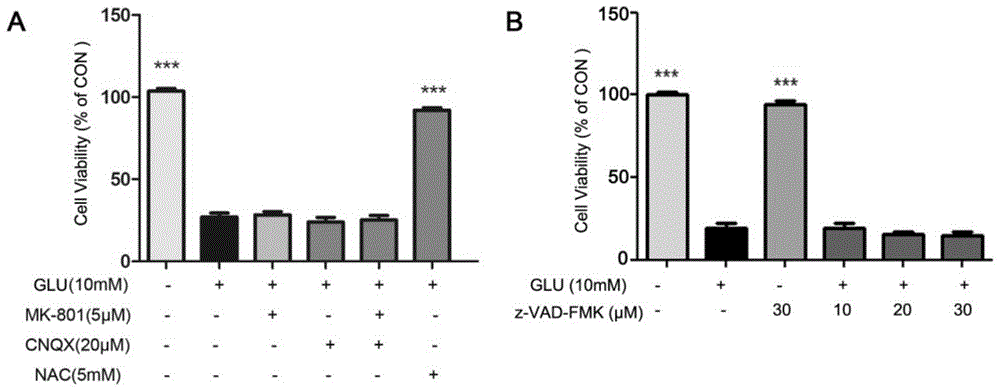
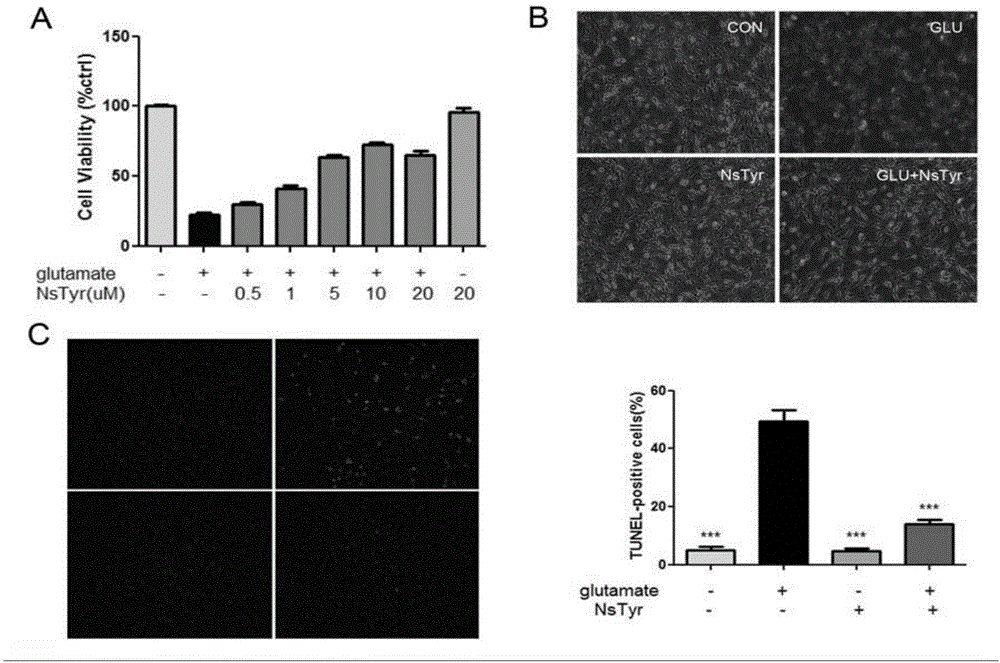
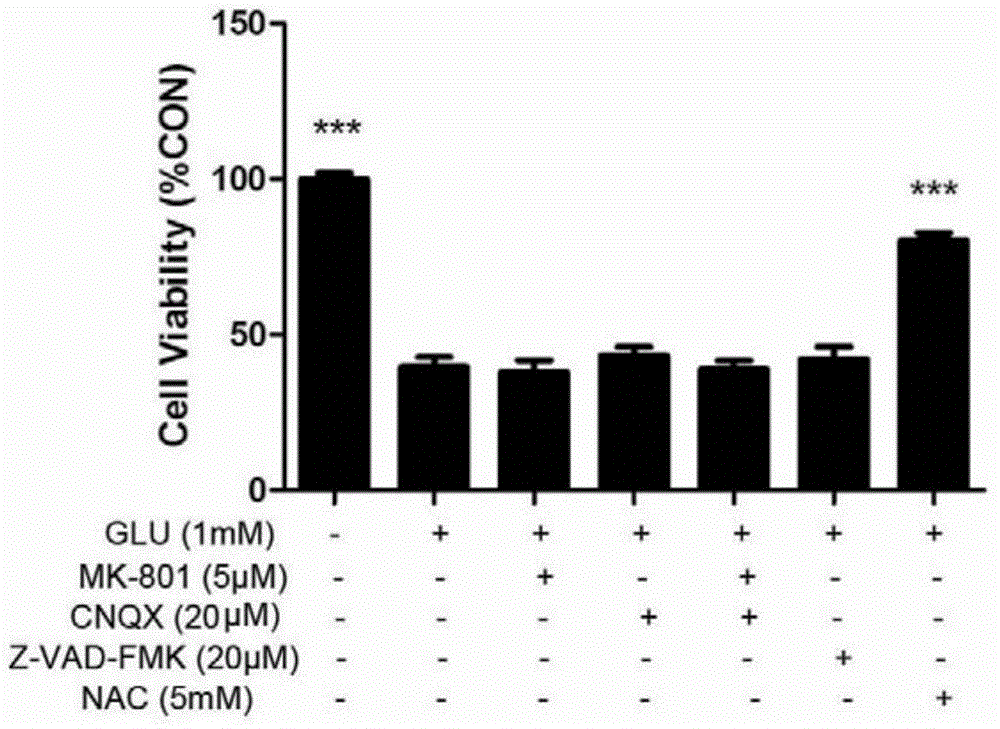
Stearoyl amino acid compound, and preparation method and applications thereof
A technology of fatty acyl amino acids and compounds, applied in the field of medicine, can solve the problem of no compound antioxidant effect, achieve significant antioxidant activity, and have broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1N-stearyl amino acid
[0048] (1) Protection of amino acids - carboxymethyl esterification
[0049] Amino acid reacts with hydrochloric acid-methanol to obtain amino acid methyl ester. Stearic acid and thionyl chloride are heated to reflux to obtain stearyl chloride.
[0050] Amino acid methyl ester was dissolved in pyridine, the above stearyl chloride was added dropwise, and the reaction was stirred overnight. After extraction, recrystallization, etc., stearyl amino acid methyl ester is obtained. Then it is hydrolyzed under alkaline conditions to obtain stearyl amino acid.
[0051]
[0052] (2) Amino acid deprotection - first activation of stearic acid (active ester method)
[0053] Stearic acid (SA) reacts with N-hydroxysuccinimide (N-HOSu) to generate activated ester, and then reacts with amino acid compounds to obtain stearamide.
[0054] Stearic acid and N-hydroxysuccinimide were stirred and reacted for 16 hours under the acti...
Embodiment 2
[0069] The preparation of embodiment 2N-stearyl amino acid
[0070] In this example, N-stearyl amino acid is prepared by a one-pot method. The one-pot method has mild reaction conditions, is not limited by amino acid solubility and system pH, and has ideal yield and purity. The following takes the one-pot preparation of N-stearyl tyrosine (NSTyr) as an example to describe the reaction steps of the one-pot method in detail.
[0071] At room temperature, with 1-hydroxybenzotriazole HOBT as coupling agent, 4-dimethylaminopyridine DMAP as catalyst, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride in One-pot synthesis of N-stearyl tyrosine methyl ester under alkaline conditions with pre-synthesized L-tyrosine methyl ester, which was hydrolyzed to N-stearyl tyrosine (NSTyr).
[0072]
[0073] 1. Preparation of Tyrosine Methyl Ester
[0074] One side of a 25ml two-necked bottle was connected to a drying tube, and the other was connected to a dropping funnel, and 5ml of...
Embodiment 3
[0077] Example 3 Structure identification
[0078] The stearyl amino acid compound that above-mentioned embodiment 1 synthesizes all passes through 1 PHNMR, 13 PCNMR identified its structure. The structural identification data of compounds 6 and 11 are listed below.
[0079] Compound 6:
[0080] White solid, mp, 106-108°C. 1HNMR (500MHz, CDCl3, TMS) δppm: 7.0 (2H, d, J=10.2Hz, H on the benzene ring); 6.7 (2H, d, J=10.5Hz, H on the benzene ring); 5.9 (1H, s, -NH-); 4.8 (1H, m, -CH-NH-); 3.1 (2H, m, -CH2-), 2.17 (2H, t, -CH2-CONH-); 1.4 (2H, m, -CH2-); 1.26 (28H, m, -(CH2)n-); 0.88 (3H, t, -CH3). 13CNMR (500MHz, CDCl3) δppm: 174.1 (-CONH-); 173.9 (-COOH); 155.3 (C of -OH on the benzene ring); 130.5, 130.3, 127.6, 121.8 and 115.8 (the other 5 C on the benzene ring ); 53.4 (-CH-NH-); 36.8 (-CH2-CONH-); 36.6 (-CH2-CHNH-); 32.0 (-CH2-); 31.6~22.6 (14×-CH2-); 14.0 (- CH3-).
[0081] The above data confirm that the compound is N-stearyl tyrosine.
[0082] Compound 11:
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com