Procyanidin A2, and preparation method and application thereof
A technology of proanthocyanidins and methanol, applied in pharmaceutical formulations, medical preparations containing active ingredients, digestive system, etc., can solve problems such as limited identification and utilization of active substances, and achieve sustainable development, wide application prospects, and inhibition of proliferation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
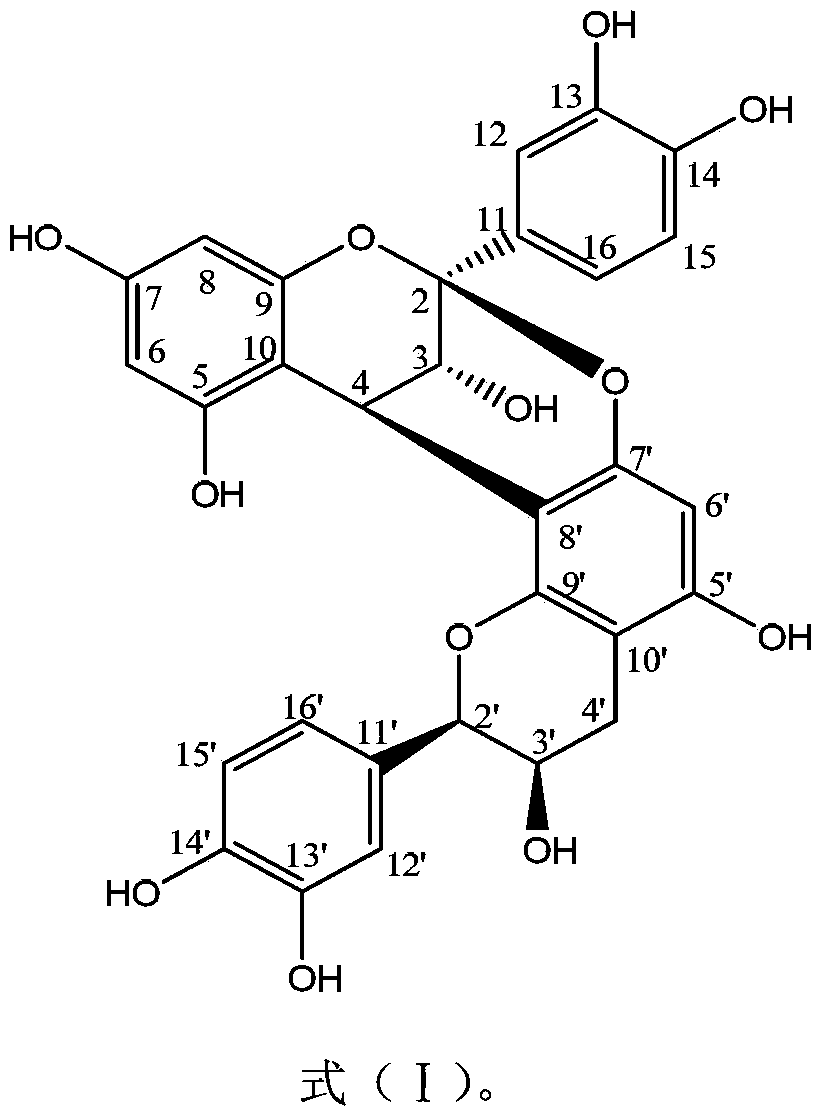
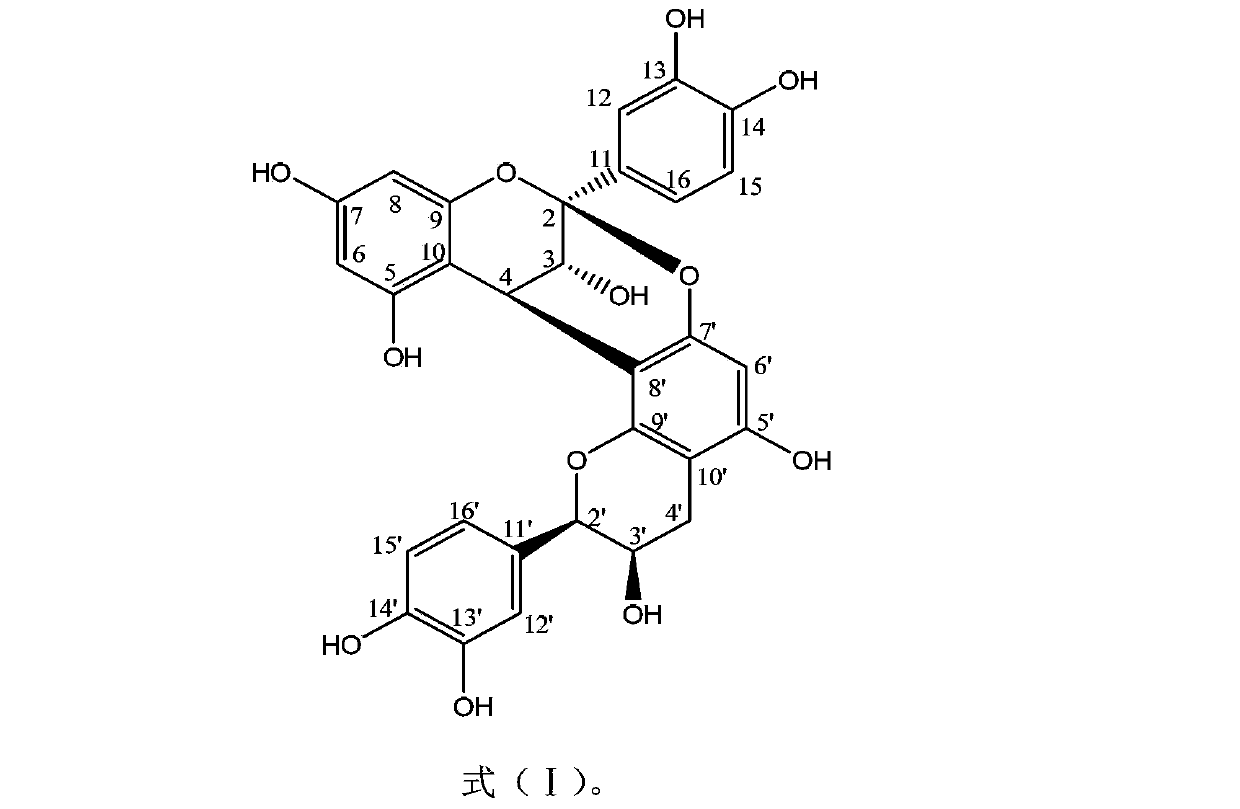
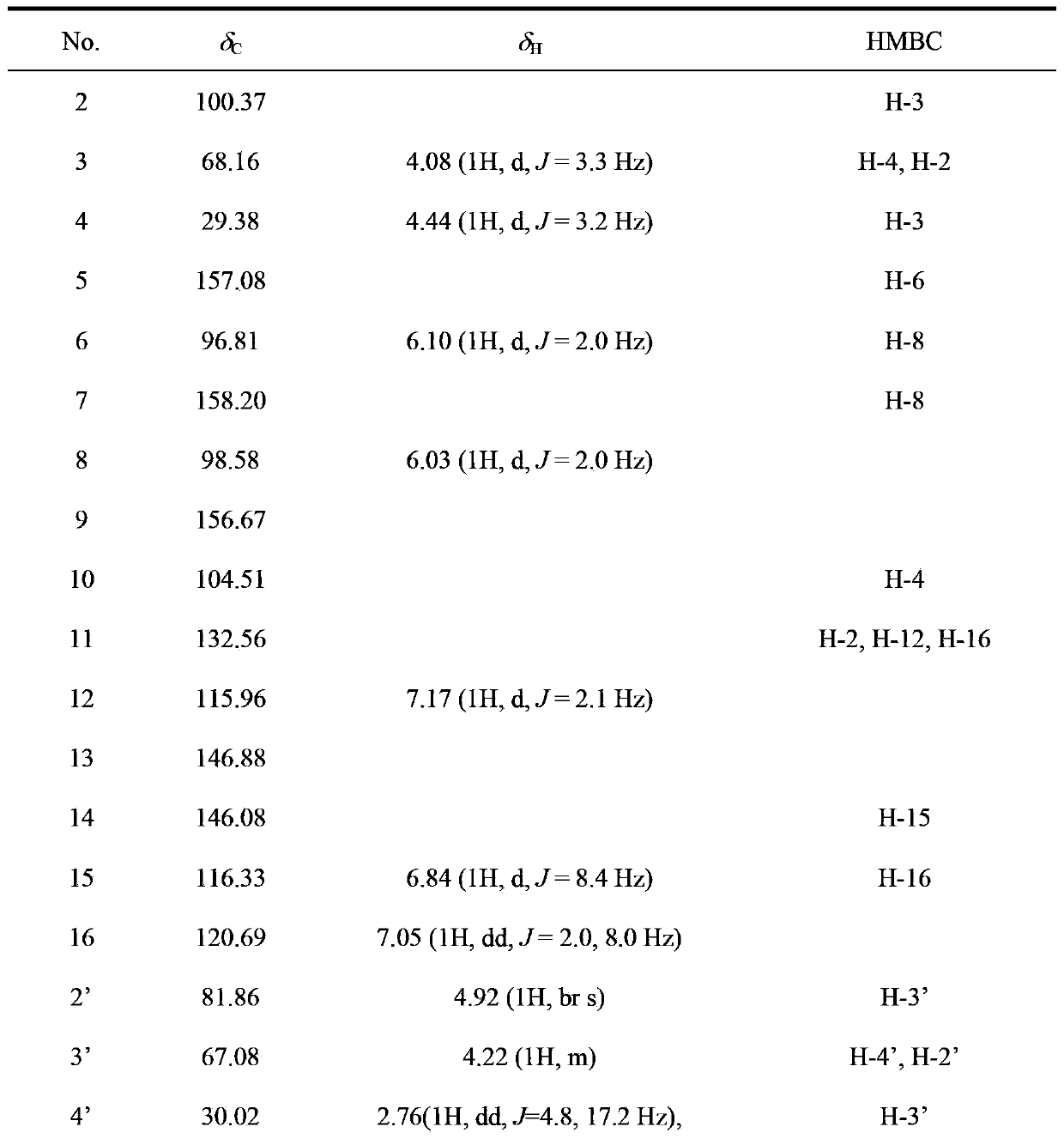
[0024] Embodiment 1: Proanthocyanidin A 2 Preparative isolation and structural elucidation.
[0025] 1. Proanthocyanidin A 2 Preparation and separation
[0026] 1) Material selection: choose fresh lychee leaves;
[0027] 2) Drying and crushing: drying or drying the lychee leaves, using a pulverizer to crush them into powder, passing through a 20-mesh sieve, collecting the sieved powder, and obtaining the lychee leaf powder;
[0028] 3) Leaching: Add 5 times the volume (5mL / g litchi leaf powder) of 40% methanol aqueous solution to the lychee leaf powder, extract at 60°C for 4 hours, filter, and collect the filtrate;
[0029] 4) Organic solvent classification: Concentrate the above filtrate at 40°C to remove methanol, add 1 times its volume of water, and successively extract three times with equal volumes of petroleum ether, ethyl acetate, and n-butanol; select ethyl acetate The extract of the ester phase was concentrated to obtain an extract for subsequent purification.
...
Embodiment 2
[0040] 1) Material selection: choose fresh lychee leaves;
[0041] 2) Drying and pulverizing: drying or drying the lychee leaves, using a pulverizer to pulverize them into powder, and passing through a 120-mesh sieve to obtain the lychee leaf powder;
[0042] 3) Extraction: add lychee leaf powder to 20 times the volume (20mL / g lychee leaf powder) of 100% methanol solution, extract at room temperature (25°C) for 30 hours, filter, and collect the filtrate;
[0043] 4) Organic solvent classification: Concentrate the above-mentioned filtrate at 60°C to remove methanol, add 3 times its volume of water, successively extract 12 times with equal volumes of petroleum ether, ethyl acetate, and n-butanol; select ethyl acetate After the ester phase extract was concentrated, the extract was obtained for subsequent purification.
[0044] Subsequent purification steps are the same as in Example 1, thus obtaining and identifying proanthocyanidin A 2 .
[0045] Proanthocyanidin A obtained b...
Embodiment 3
[0047] 1) Material selection: choose fresh lychee leaves;
[0048]2) drying and crushing: dry or dry the lychee leaves, use a pulverizer to pulverize them into powder, and pass through a 60-mesh sieve to obtain the lychee leaf powder;
[0049] 3) Extraction: Add 10 times the volume of litchi leaf powder (10mL / g litchi leaf powder) to a 40% ethanol aqueous solution, extract at 40°C for 15 hours, filter, and collect the filtrate;
[0050] 4) Organic solvent classification: Concentrate the above-mentioned filtrate at 60°C to remove ethanol, add 2 times the volume of water, and successively extract 6 times with equal volumes of petroleum ether, ethyl acetate, and n-butanol; After the ester phase extract was concentrated, the extract was obtained for subsequent purification.
[0051] Subsequent purification steps are the same as in Example 1, and are obtained and identified as proanthocyanidin A 2 .
[0052] Proanthocyanidin A obtained by this method 2 The yield is 2.25 ~ 3.58g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com