Application of irisin in preparation of antihyperglycemic medicines
An irisin and anti-diabetic technology, applied in the field of medicine, can solve the problems such as the anti-diabetic drug effect of irisin that has not been reported, and achieve the effect of broadening the selection field.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
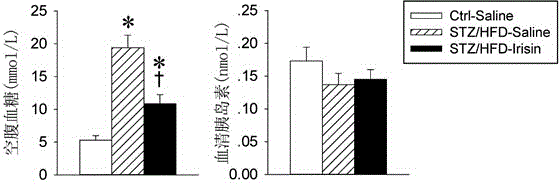
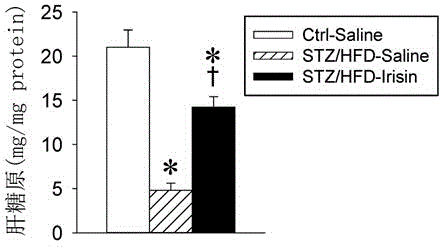
[0016] Embodiment 1 The making of diabetes model
[0017] Six-week-old C57BL / 6J mice were randomly divided into three groups:
[0018] (1) Normal mice-physiological saline group (Ctrl-Saline): as the normal control group, they were given a normal diet (non-high-fat diet) all the time, and after 8 weeks, they were given subcutaneous infusion of saline (saline) for 2 weeks as a control of irisin ;
[0019] (2) Diabetes-saline group (STZ / HFD-Saline): Diabetes was induced by streptozotocin (STZ) combined with high-fat diet (HFD), and after 8 weeks, normal saline (saline) was subcutaneously infused for 2 weeks as irisin contrast;
[0020] (3) Diabetes-irisin group (STZ / HFD-Irisin): Diabetes was induced by Streptozotocin (STZ) combined with high-fat diet (HFD), and irisin was subcutaneously infused for 2 weeks after 8 weeks.
[0021] Diabetes model making method: The diabetes model is induced by combined application of small doses of STZ and high-fat diet, which is a recognized...
Embodiment 2
[0022] Example 2 Subcutaneous continuous administration of micro-osmotic pump
[0023] Under pentobarbital sodium anesthesia and aseptic operation, a miniature osmotic pressure pump (1002 type, Alzet company) containing normal saline or irisin was implanted into the subcutaneous tissue of the back of the mouse neck, the incision was sutured, and penicillin was injected intramuscularly every day after the operation 3 days in a row to prevent infection. The infusion dose of irisin was 1.44 nmol / day (0.24 nmol / μl) for 2 consecutive weeks.
Embodiment 3
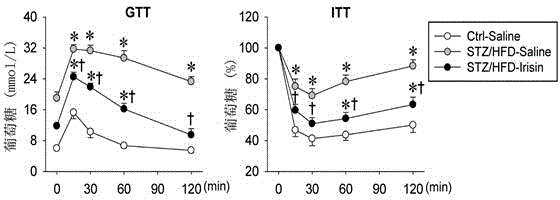
[0024] Example 3 Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
[0025] Glucose tolerance test (GTT): After the mice were fasted overnight, glucose (2.0 g / kg) was injected intraperitoneally, and blood glucose was measured before and 15, 30, 60 and 120 min after the injection.
[0026] Insulin tolerance test (insulin tolerance test, ITT): mice were fasted for 6 hours, and insulin (0.75 units / kg) was injected intraperitoneally, and blood glucose was measured before and 15, 30, 60 and 120 minutes after injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com