Synthetic method of 2-(5-fluoro-2,4-dinitrophenoxy)acetate
A technology of dinitrophenoxy and a synthesis method, applied in the field of 2-acetate synthesis, can solve problems such as high production cost, difficult separation and purification, and achieve the effects of less side reactions, good selectivity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
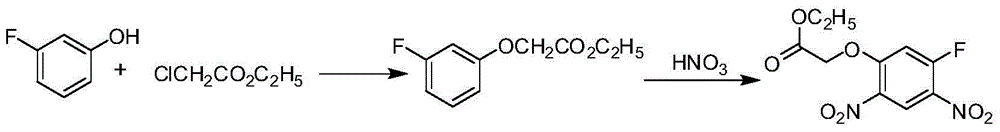
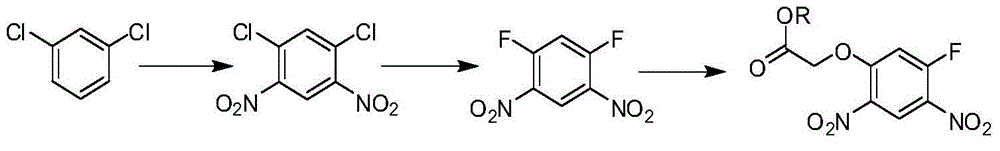
[0024] The synthetic method of 2-(5-fluoro-2,4-dinitrophenoxy) acetate comprises the following steps:
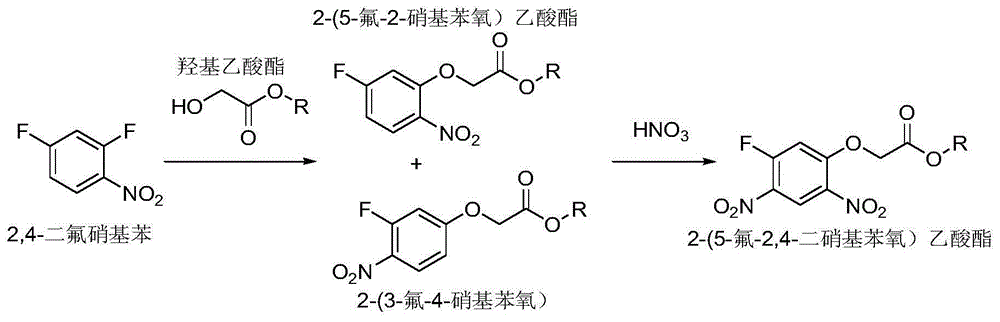
[0025] a, 2,4-difluoronitrobenzene reacts with glycolate under the action of an acid-binding agent to obtain 2-(5-fluoro-2-nitrophenoxy) acetate and 2-(3-fluoro -A mixture of 4-nitrophenoxy)acetate; the reaction temperature is 40-120°C, and the reaction time is 5-20h; the amount of 2,4-difluoronitrobenzene and glycolate The ratio is 1:1-1.2; the ratio of the amount of 2,4-difluoronitrobenzene to the acid-binding agent is 1:1-2.5;
[0026] b. React the mixture obtained in step a with nitric acid to obtain 2-(5-fluoro-2,4-dinitrophenoxy)acetate; the reaction temperature is -10°C to 10°C, and the reaction time is 30min ~2h; the ratio of the mixture to the amount of nitric acid is 1:1~1.2.
[0027] In the above-mentioned synthetic method of 2-(5-fluoro-2,4-dinitrophenoxy) acetate, the general formula of the glycolic acid ester described in step a is CH 2 (OH)COOR, where R is ...
Embodiment 1
[0031] Example 1 Synthesis of 2-(5-fluoro-2,4-dinitrophenoxy)methyl acetate
[0032] Add 2,4-difluoronitrobenzene (20.4g, 0.1mol), anhydrous potassium carbonate (20.73g, 0.15mol), tetrahydrofuran 102g into the 500mL reaction flask, heat to reflux and slowly drop into methyl glycolate (10.42 g, 0.115mol). After the dropwise addition, continue to reflux for 6 hours, filter and concentrate to obtain methyl 2-(5-fluoro-2-nitrophenoxy)acetate (85%), methyl 2-(3-fluoro-4-nitrophenoxy)acetate (12%) mixture, with a total weight of 22.8g and a yield of 99.5%, was directly used in the next reaction.
[0033] Add 2-(5-fluoro-2-nitrophenoxy)methyl acetate, a mixture of 2-(3-fluoro-4-nitrophenoxy)methyl acetate (22.9g, 0.1mol) into the 500mL reaction flask , 91.6g of dichloroethane, 22.9g of concentrated sulfuric acid, cooled to 0-5°C and slowly added fuming nitric acid (6.3g, 0.1mol) dropwise and continued to react for 30 minutes, separated phases, continued to use dichloroethane Extra...
Embodiment 2
[0035] Example 2 Synthesis of 2-(5-fluoro-2,4-dinitrophenoxy) ethyl acetate
[0036] The methyl glycolate in example 1 is replaced with ethyl glycolate (11.96g, 0.115mol), and the same steps as in Example 1 are adopted to obtain 2-(5-fluoro-2-nitrophenoxy) ethyl acetate A mixture of ester (88%) and ethyl 2-(3-fluoro-4-nitrophenoxy)acetate (10%), total weight 24g, yield 98.4%. Then the mixture was nitrated to finally obtain 26.4 g of solid ethyl 2-(5-fluoro-2,4-dinitrophenoxy)acetate, with a content of 95% and a yield of 91.2%.
[0037] After recrystallization with ethanol, a pure product with a content greater than 98% was obtained, mp: 58.8-59.3°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com