Group A streptococcus drug for injection and preparation method of group A streptococcus drug
A group A streptococcus, injection technology, applied in the field of medicine and biology, can solve the problems of drug failure, increase the side effects of clinical use, easy death of bacteria, etc., and achieve the effect of restoring physiological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The following formulations take 10,000 pieces as an example, each with 1KE specifications:
[0040] a. Group A Streptococcus freezing tube activation and blood slant culture: take a freeze-dried tube, directly add sterile saline to dissolve, insert into 5% yeast powder medium and activate at 37±0.5°C for 18-24 hours , and then smear microscopic examination, after confirming that streptococcus grows normally and without pollution, put the strain into the blood agar slant and incubate at 37±0.5°C for 18-24 hours;
[0041] b. Inoculation culture: adopt the three-level culture method, the first-level culture system inoculates the bacteria from the blood slant into the first-level yeast extract powder medium and cultivates it at 37±0.5°C for 20 hours, and the culture solution is checked by smear microscope. After confirming that the streptococcus grows normally and has no pollution, insert it into the secondary yeast extract powder medium according to the inoculation amount ...
Embodiment 2
[0052] Also take the preparation of 10,000 bottles as an example, each with a specification of 1KE: only when adding sterile methionine solution to 10,000 ml in step d, the concentration of the added sterile methionine solution is 2%, and the rest of the steps are the same as in Example 1 . The test results of three batches of finished products are as follows:
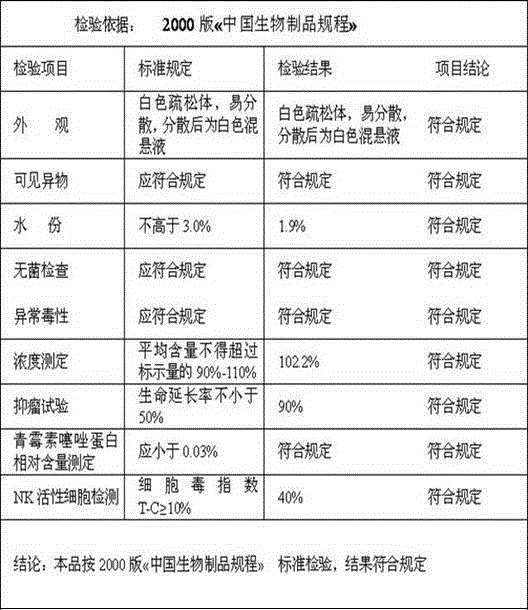
[0053] Batch number: 20140517 Specification: 1KE
[0054]
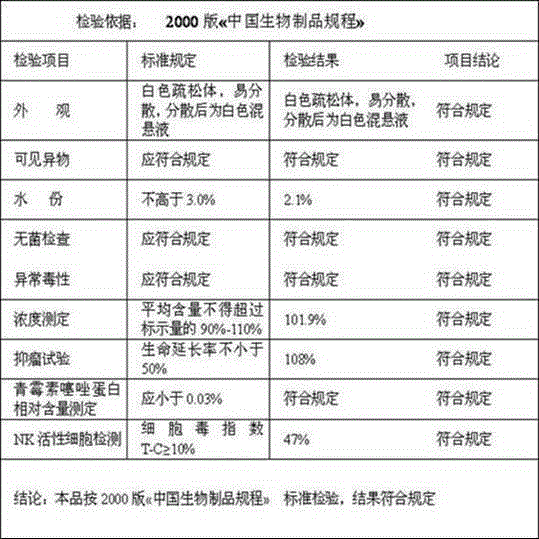
[0055] Batch number: 20140518 Specification: 1KE
[0056]
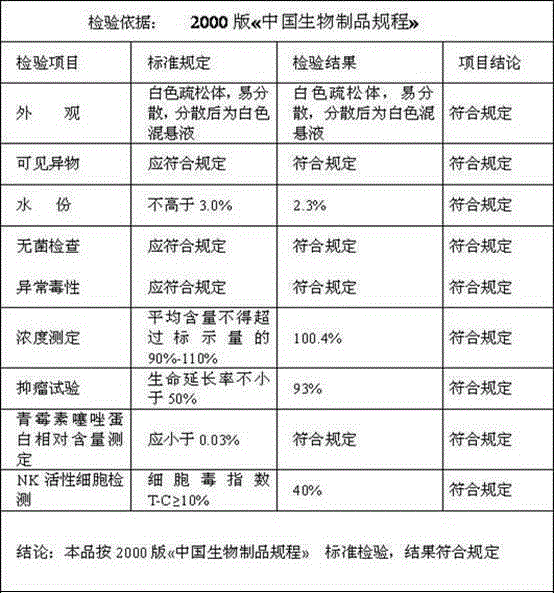
[0057] Batch number: 20140519 Specification: 1KE
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com