Preparation method of group A streptococcus medicine for injection
A group A streptococcus, injection technology, applied in the field of medicine and biology, can solve the problems of drug failure, increased clinical side effects, bacterial death and other problems, and achieve the effect of restoring physiological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The following formulations take 10,000 pieces as an example, each with 1KE specifications:
[0040] a. Group A Streptococcus freezing tube activation and blood agar slant culture: take a freeze-dried tube, directly add sterile saline to dissolve, insert 5% yeast powder medium and activate at 37±0.5°C for 18-24 hours, then smear microscopic examination, after confirming that the streptococcus grows normally and without contamination, put the strains into the blood agar slant and incubate at 37±0.5°C for 18-24 hours;
[0041] b. Inoculation culture: adopt the three-level culture method, the first-level culture system is to inoculate the bacteria from the blood agar slant into the first-level yeast extract powder medium and cultivate it at 37±0.5°C for 20 hours, and the culture solution is examined by smear microscope After confirming that the streptococcus grows normally and has no pollution, insert the inoculation amount of 5% v / v into the secondary yeast extract powder ...
Embodiment 2
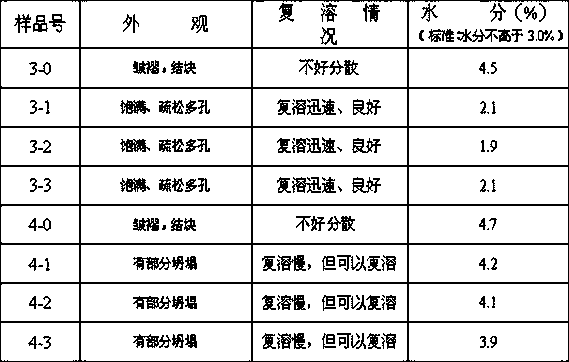
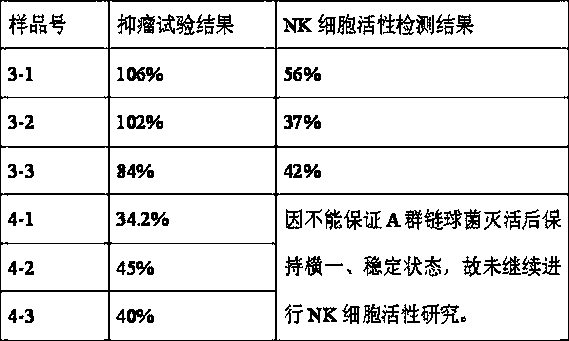
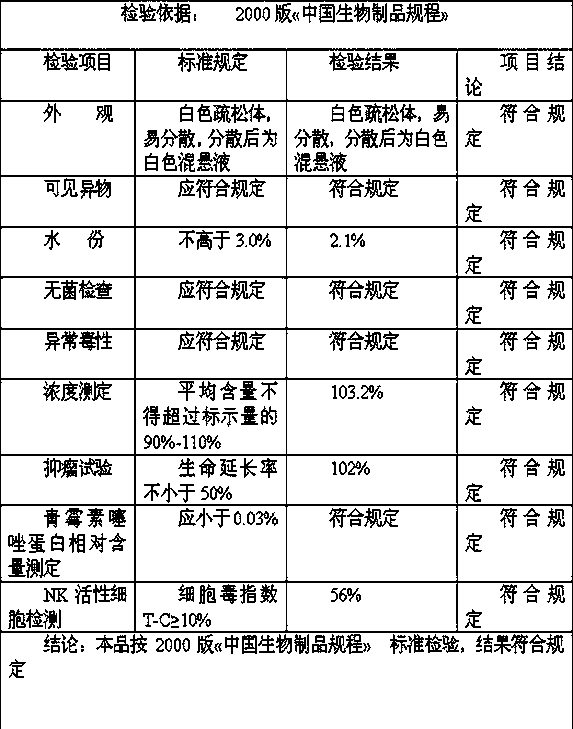
[0052] Also take the preparation of 10,000 bottles as an example, each with a specification of 1KE: only when adding sterile methionine solution to 10,000 ml in step d, the concentration of the added sterile methionine solution is 2%, and the rest of the steps are the same as in Example 1 . The test results of three batches of finished products are as follows:
[0053] Batch number: 20140517 Specification: 1KE
[0054]
[0055] Batch number: 20140518 Specification: 1KE
[0056]
[0057] Batch number: 20140519 Specification: 1KE
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com