Patents
Literature
362 results about "Time critical" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
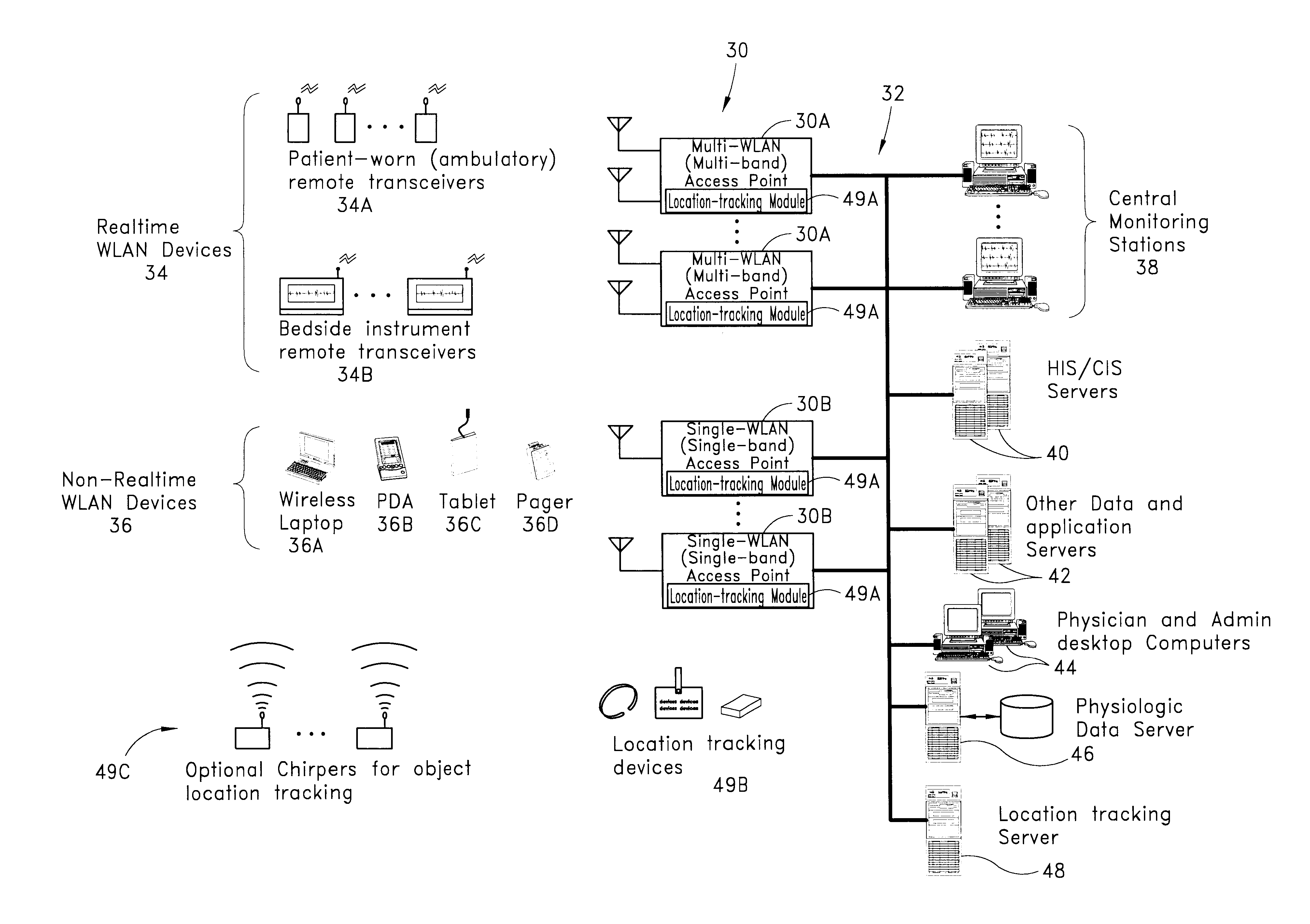
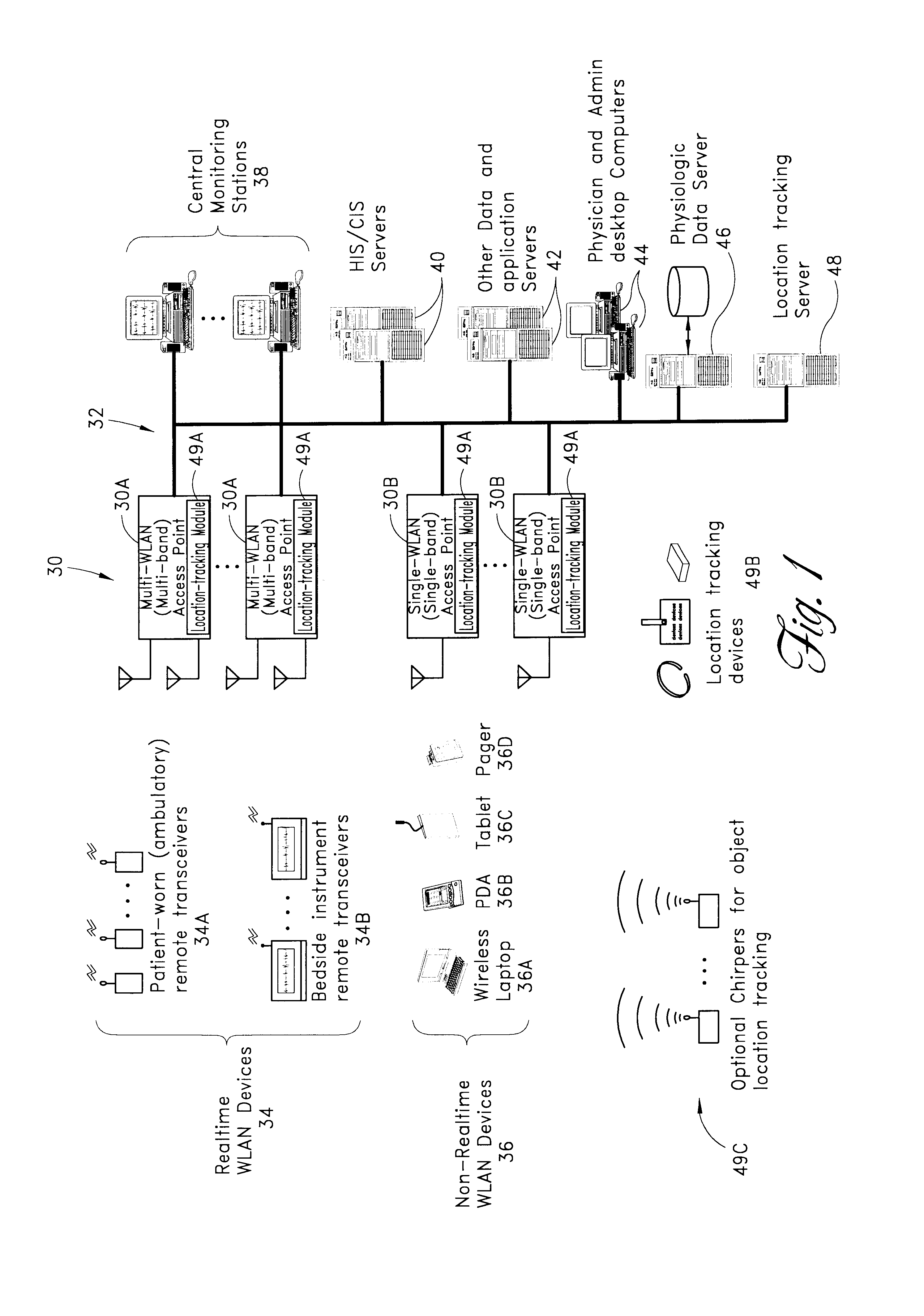
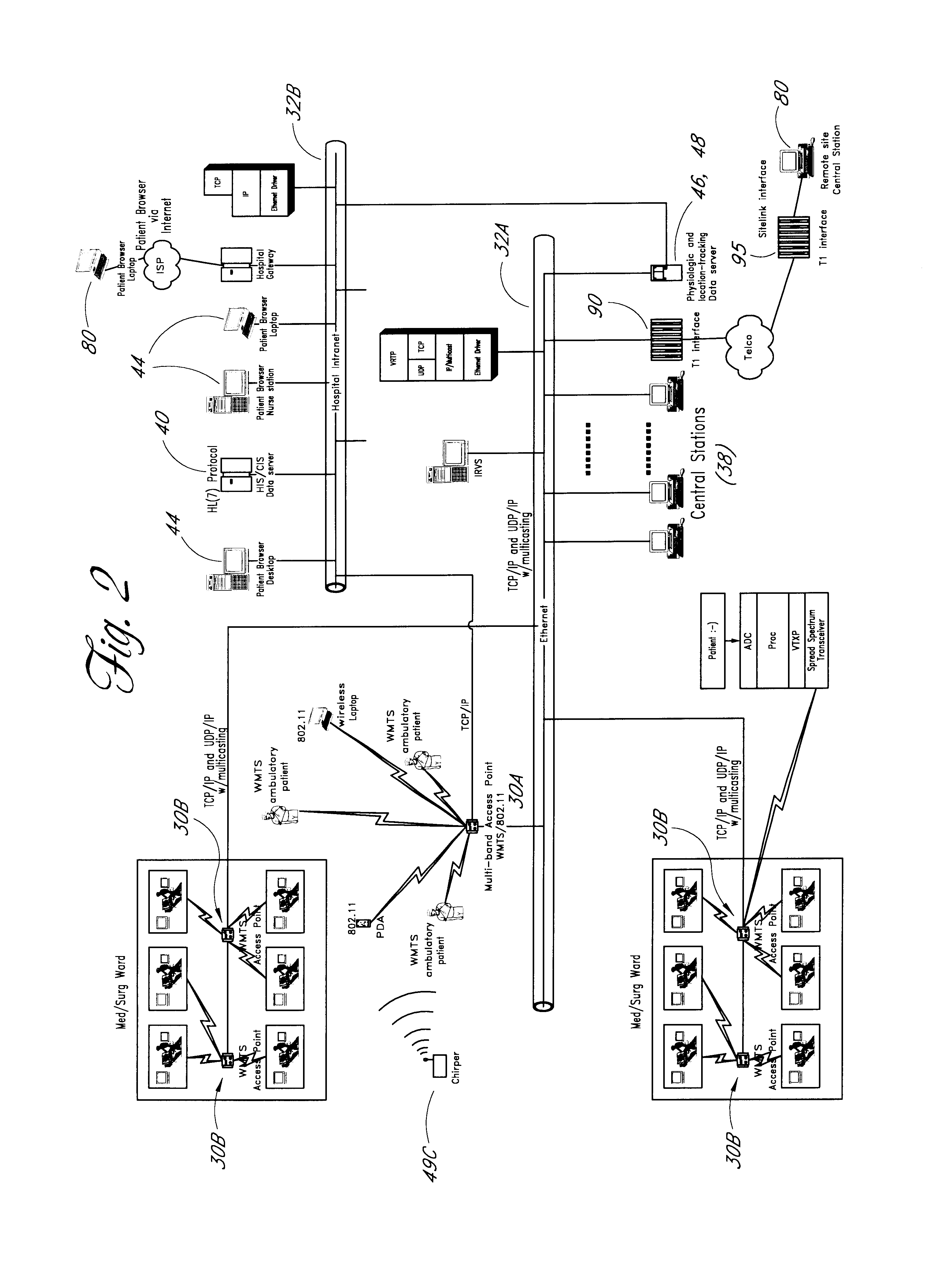
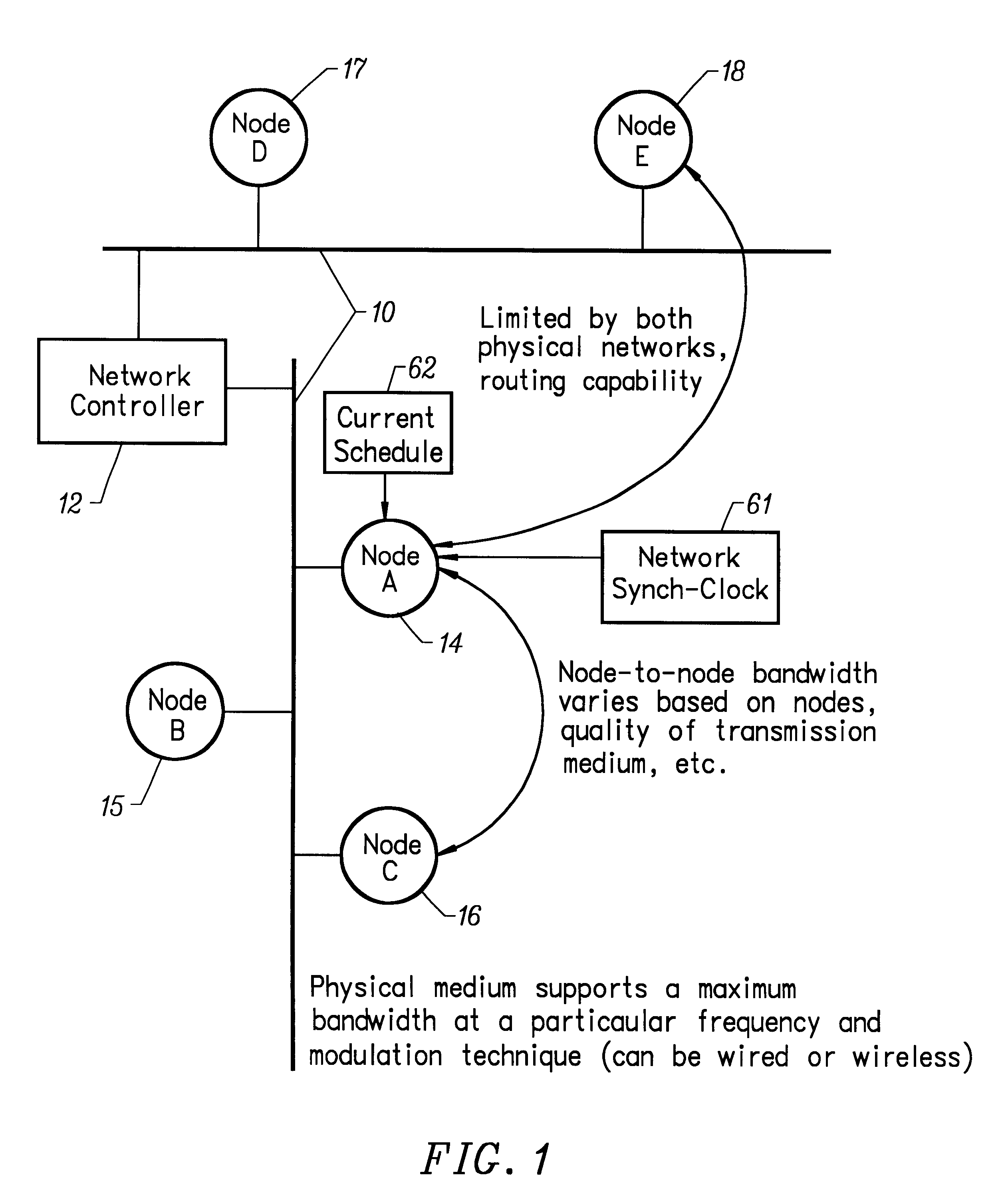
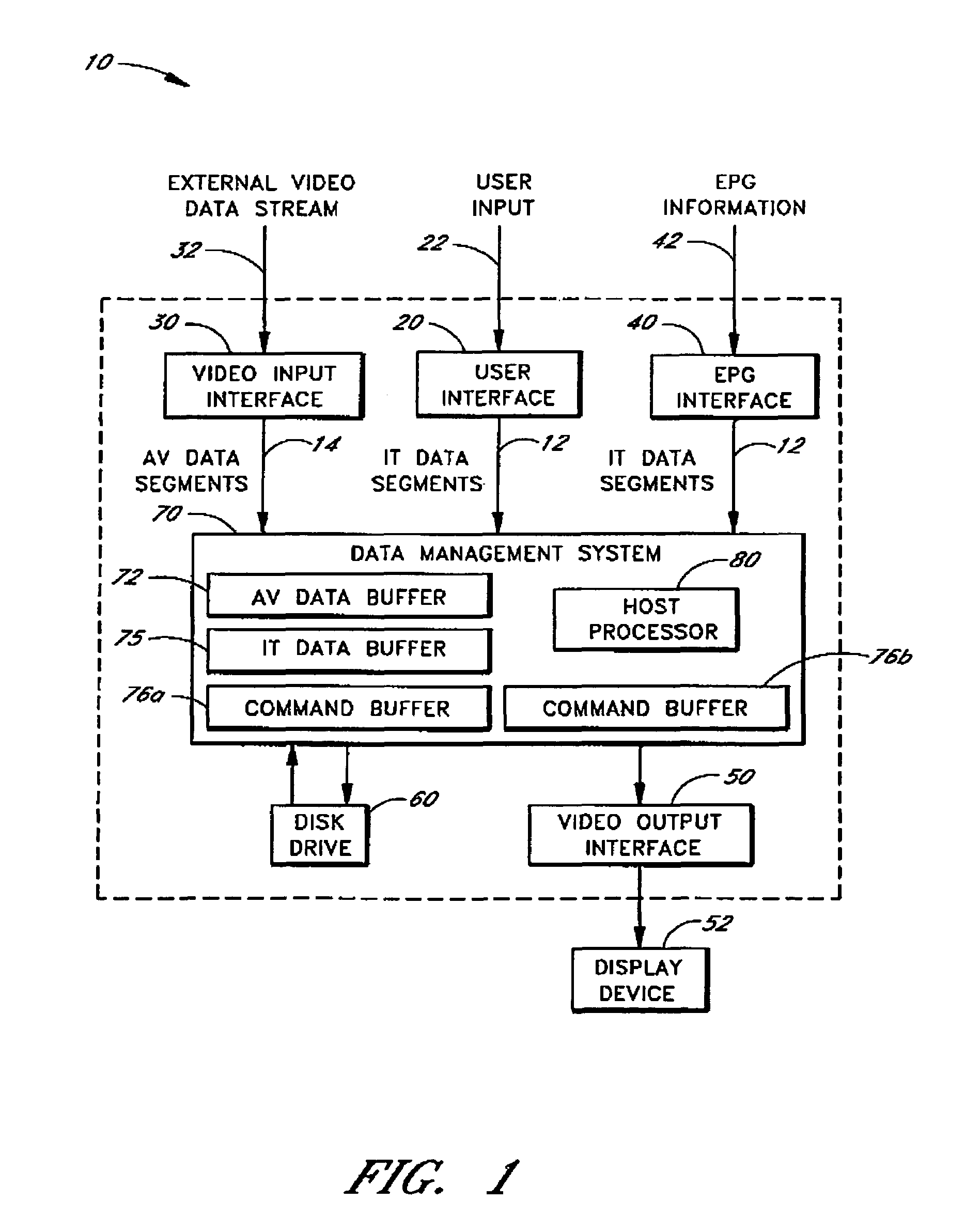
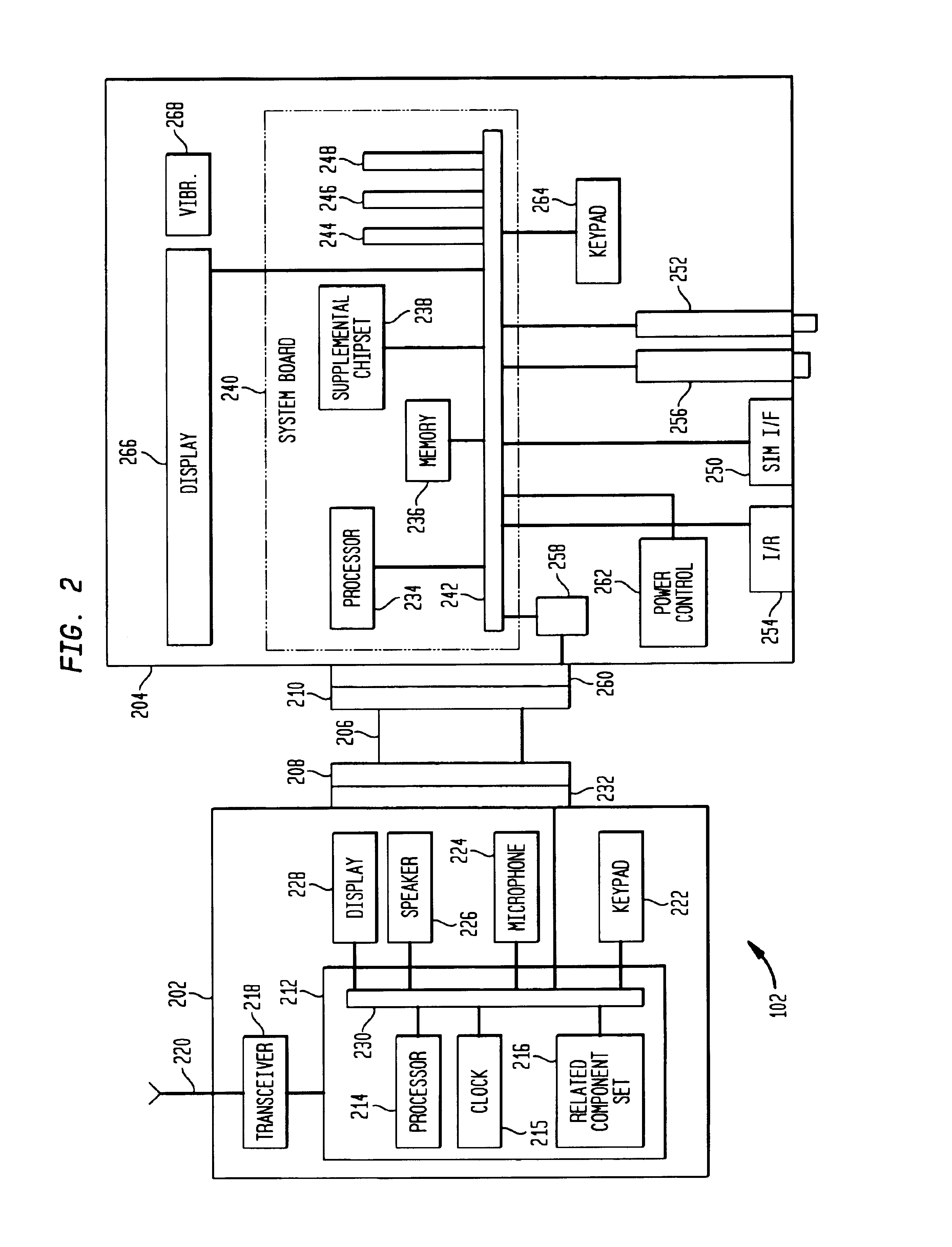
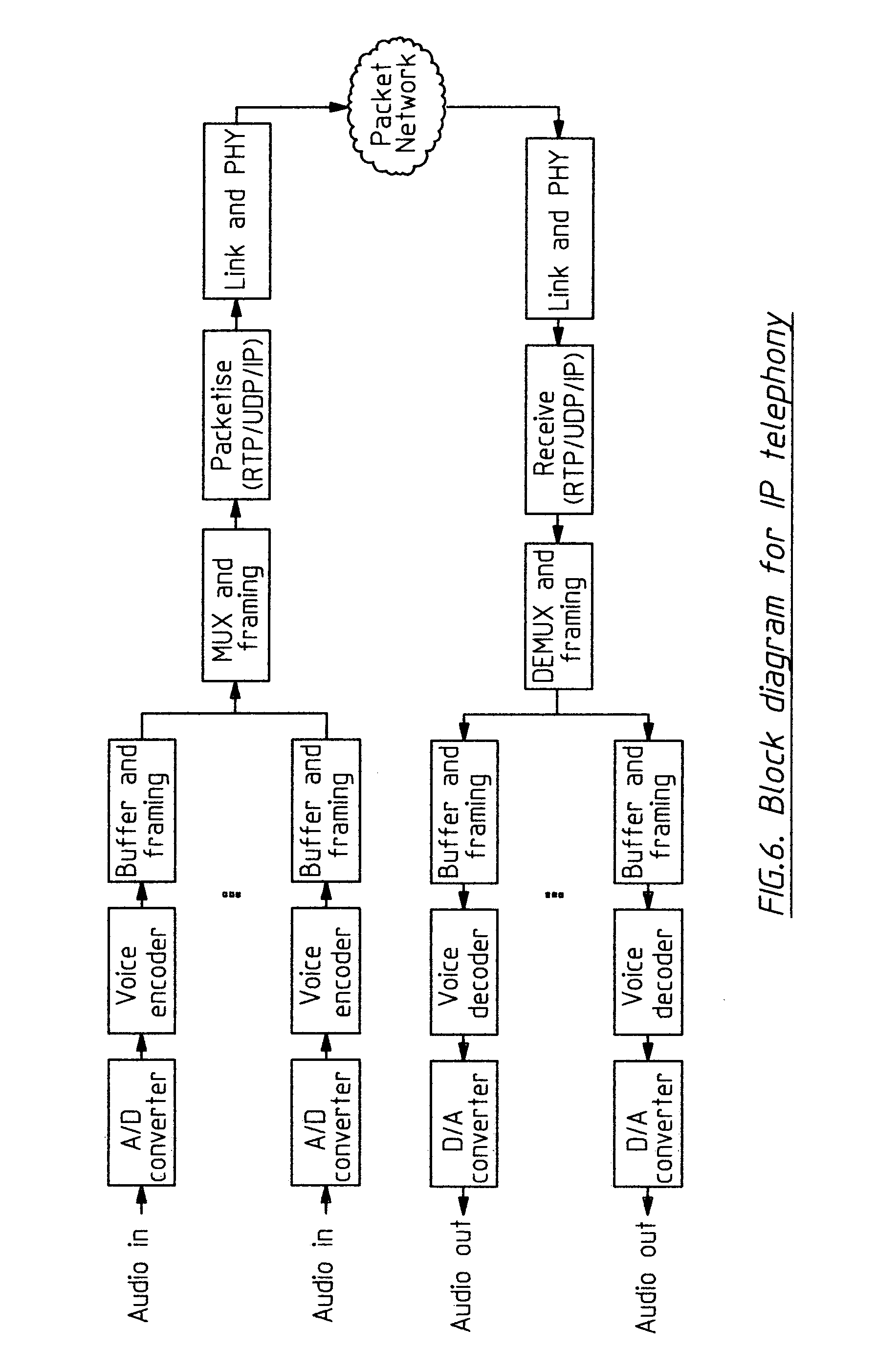
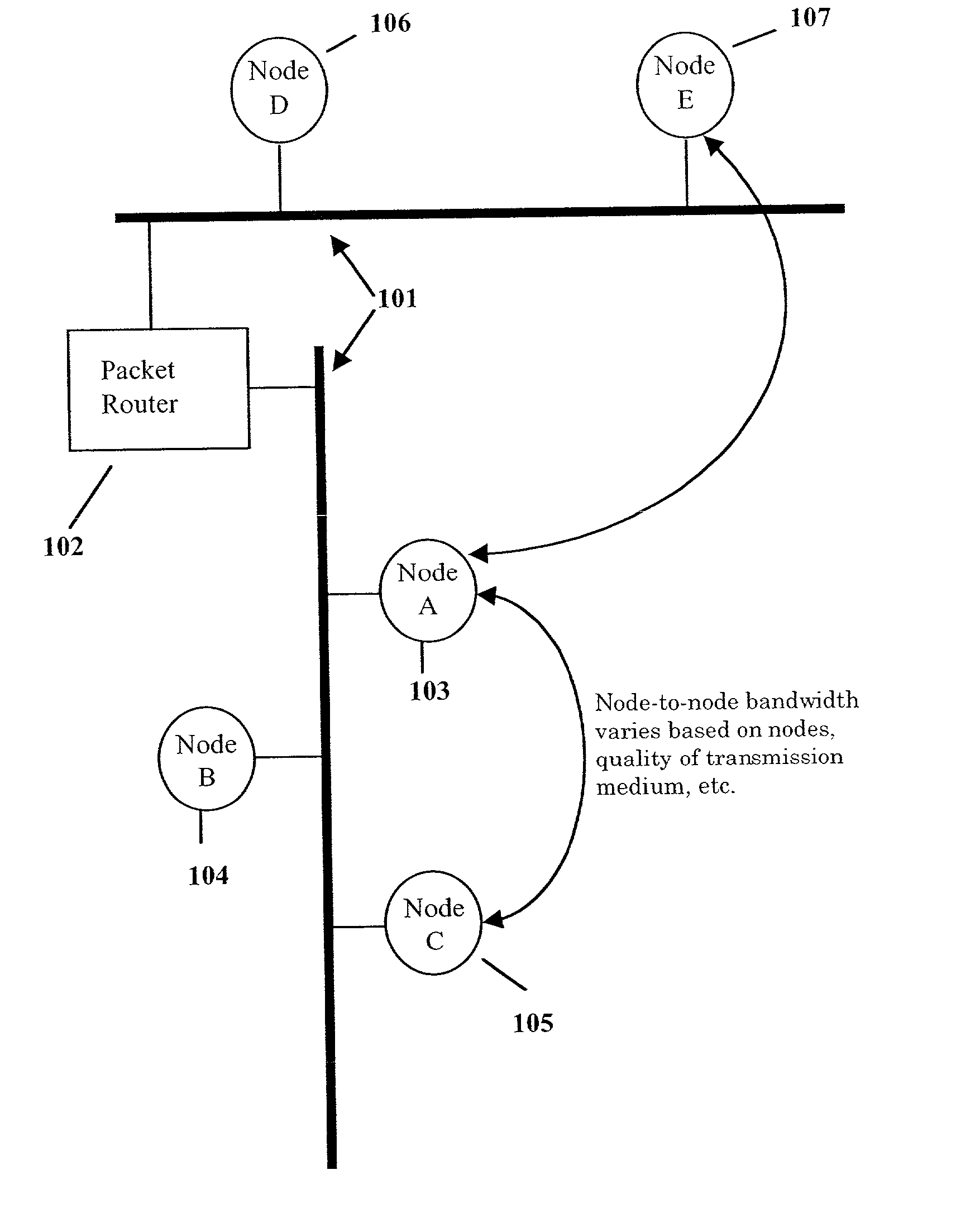
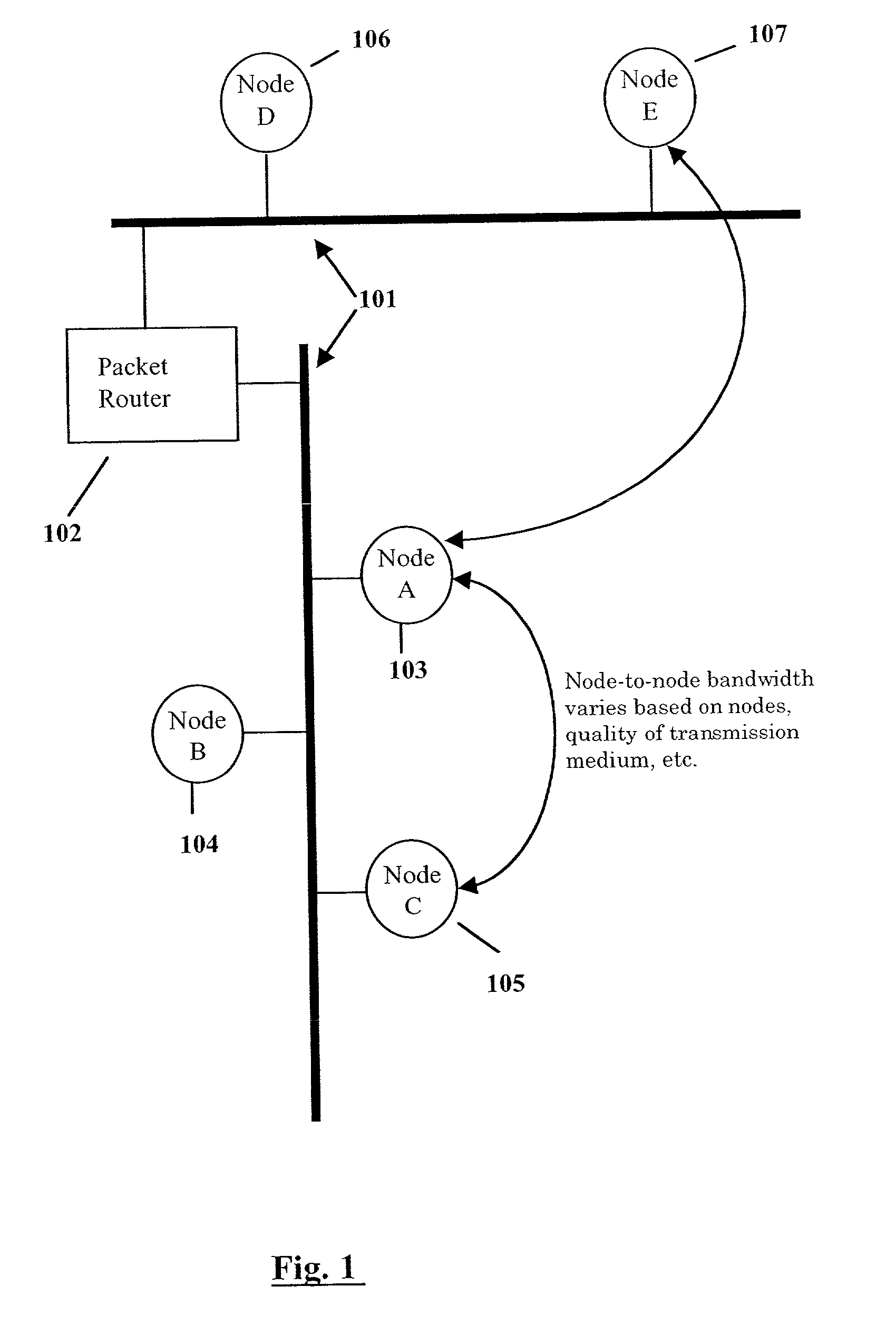
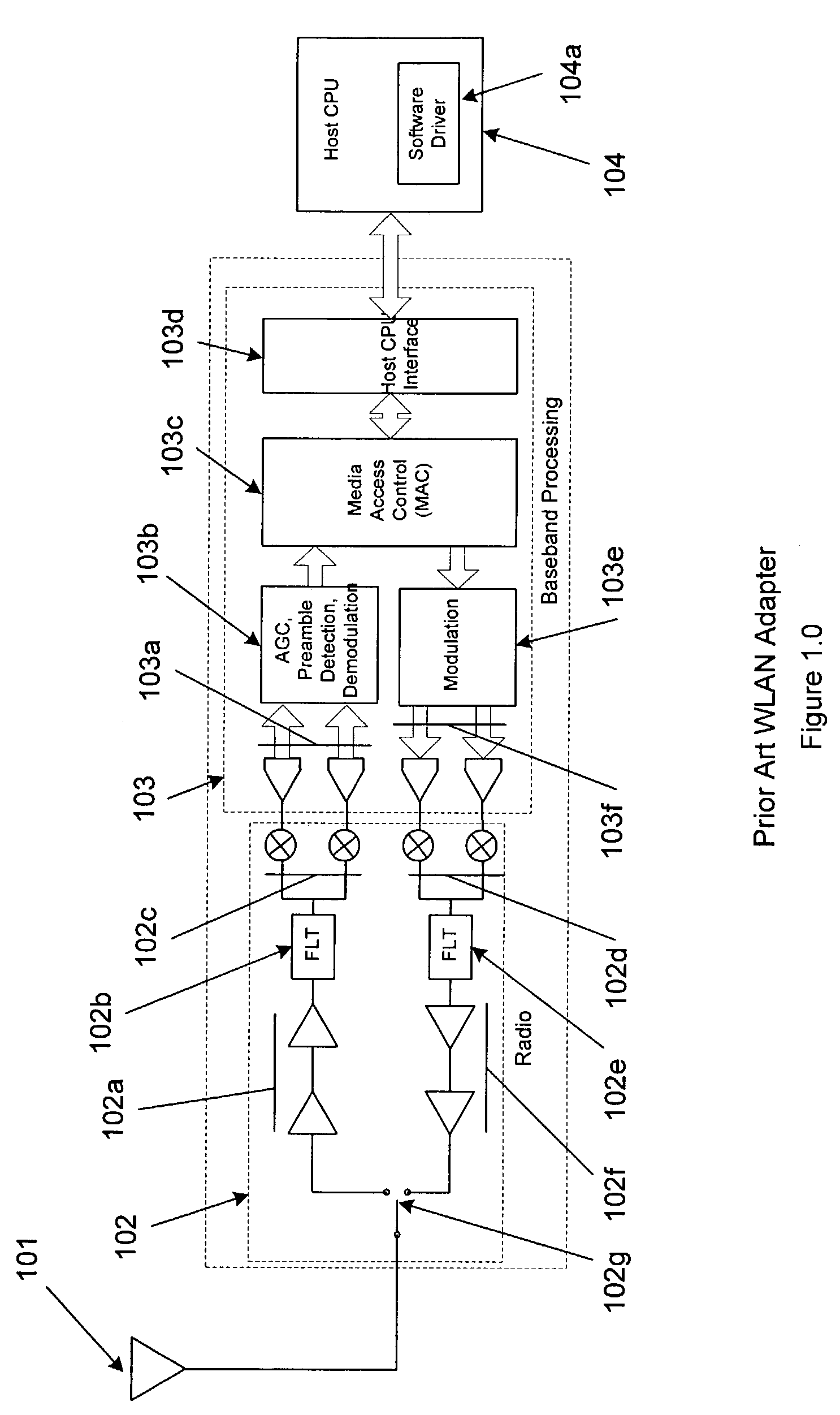
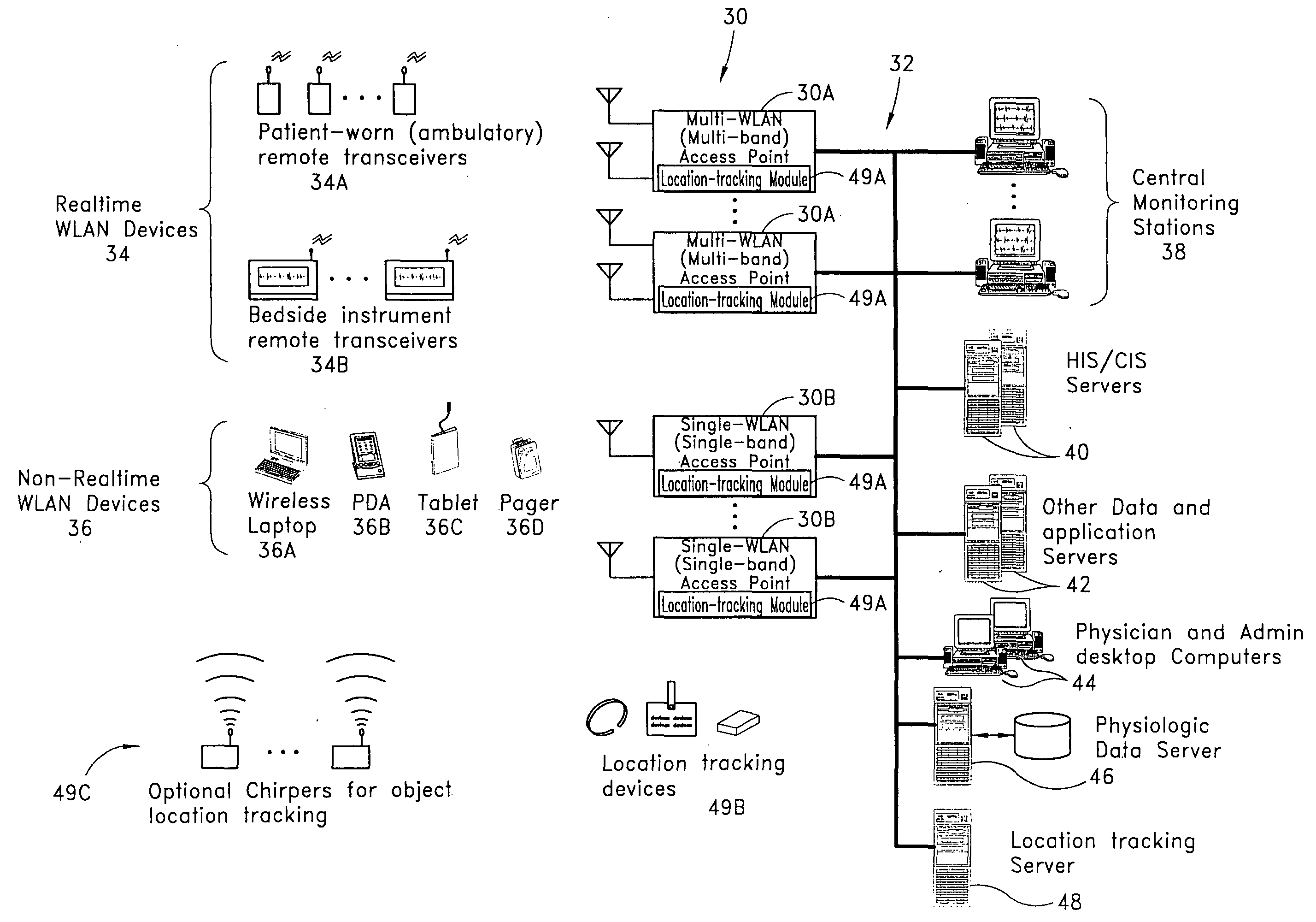
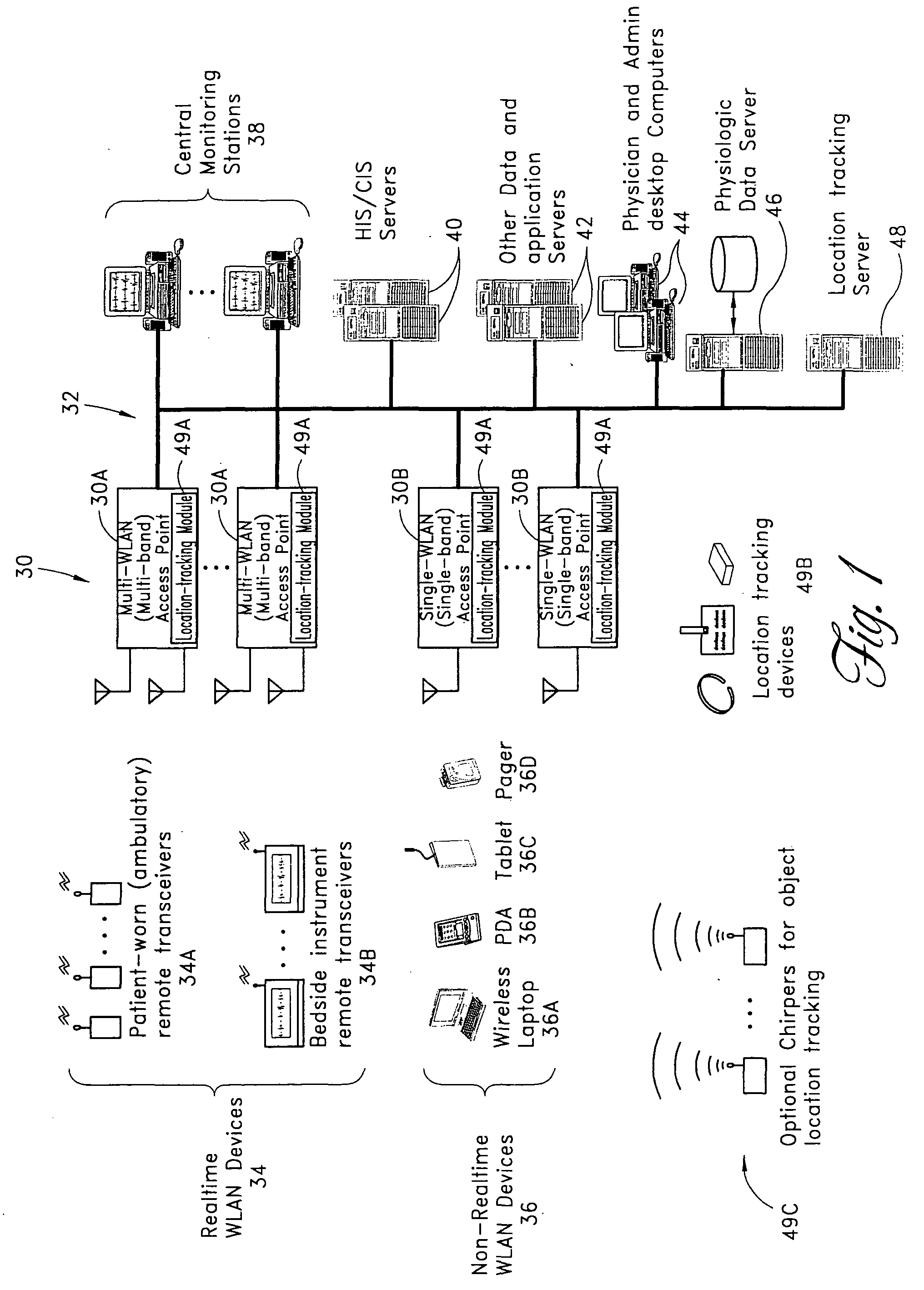
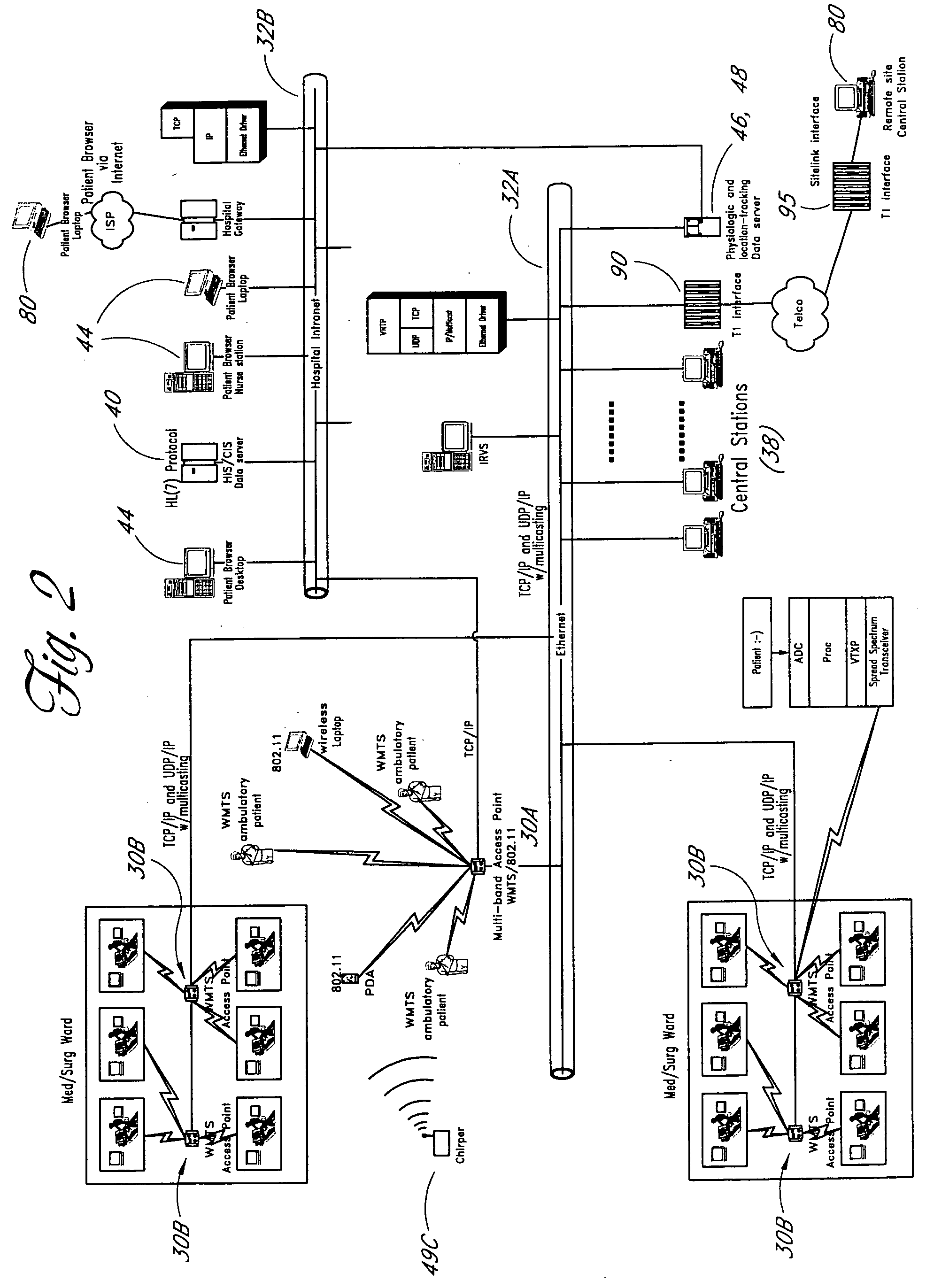
Wireless LAN architecture for integrated time-critical and non-time-critical services within medical facilities
InactiveUS6659947B1Avoiding lockstep interferenceEasy to receiveSurgeryData switching by path configurationAntenna designDevice type
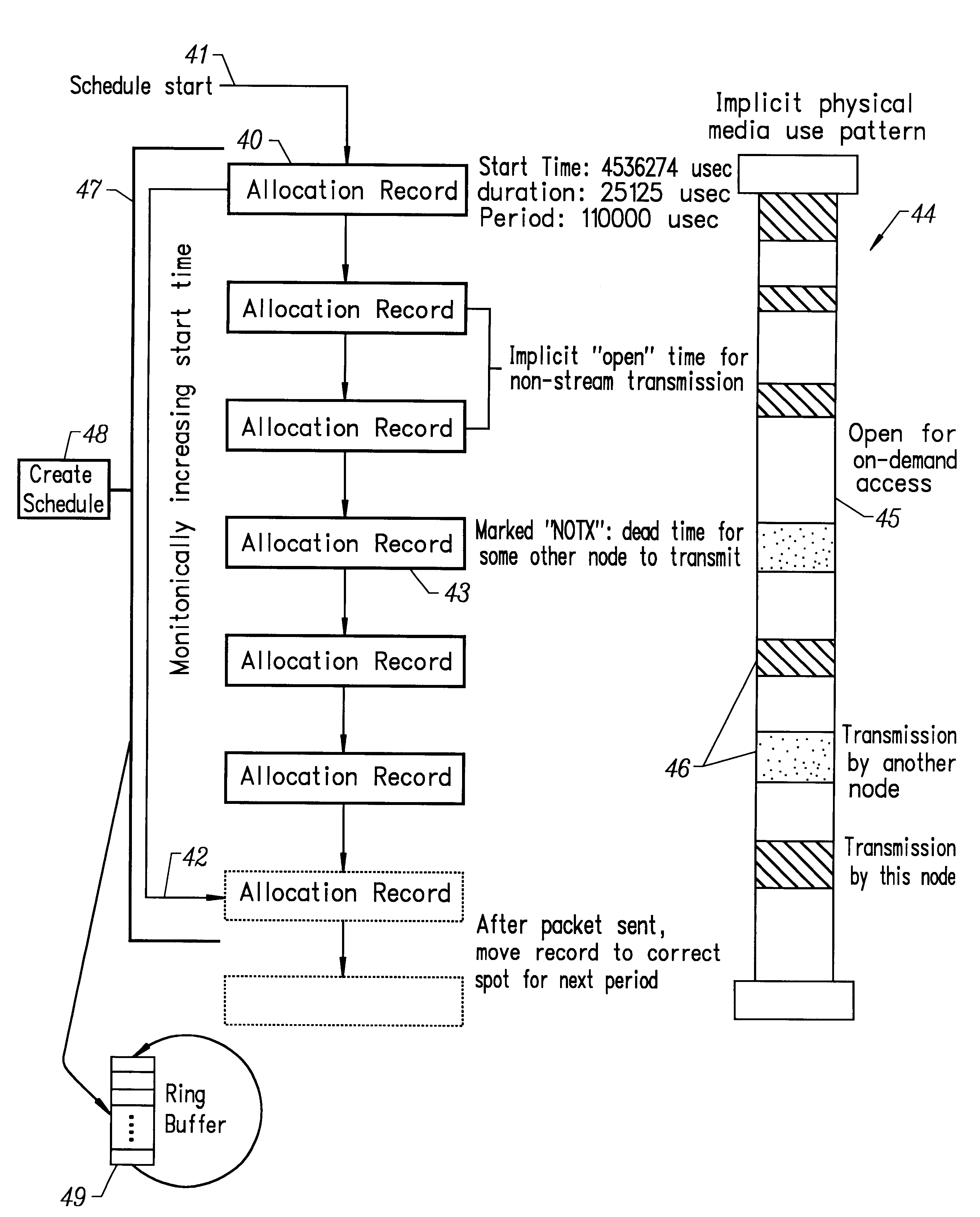
A wireless local area network (WLAN) system comprises multiple access points that are distributed throughout a medical facility to provide wireless access to a hardwired network. The access points implement multiple WLAN protocols, including a realtime protocol for realtime patient monitoring (telemetry) and a standard WLAN protocol (such as IEEE 802.11 within an ISM band) for providing general-purpose wireless access. Some or all of the access points preferably implement both WLAN protocols such that the different WLANs and wireless device types share network access resources. Some or all of the access points may also include RF location-tracking modules which may be used to track locations of patients, hospital personnel, capital equipment, and / or disposable medical supplies. Also disclosed are an antenna design which may be used with the access points to improve reception (particularly for patient monitoring), and a TDMA timeslot rotation method for avoiding lockstep interference between access points that operate on the same channel.
Owner:GE MEDICAL SYST INFORMATION TECH
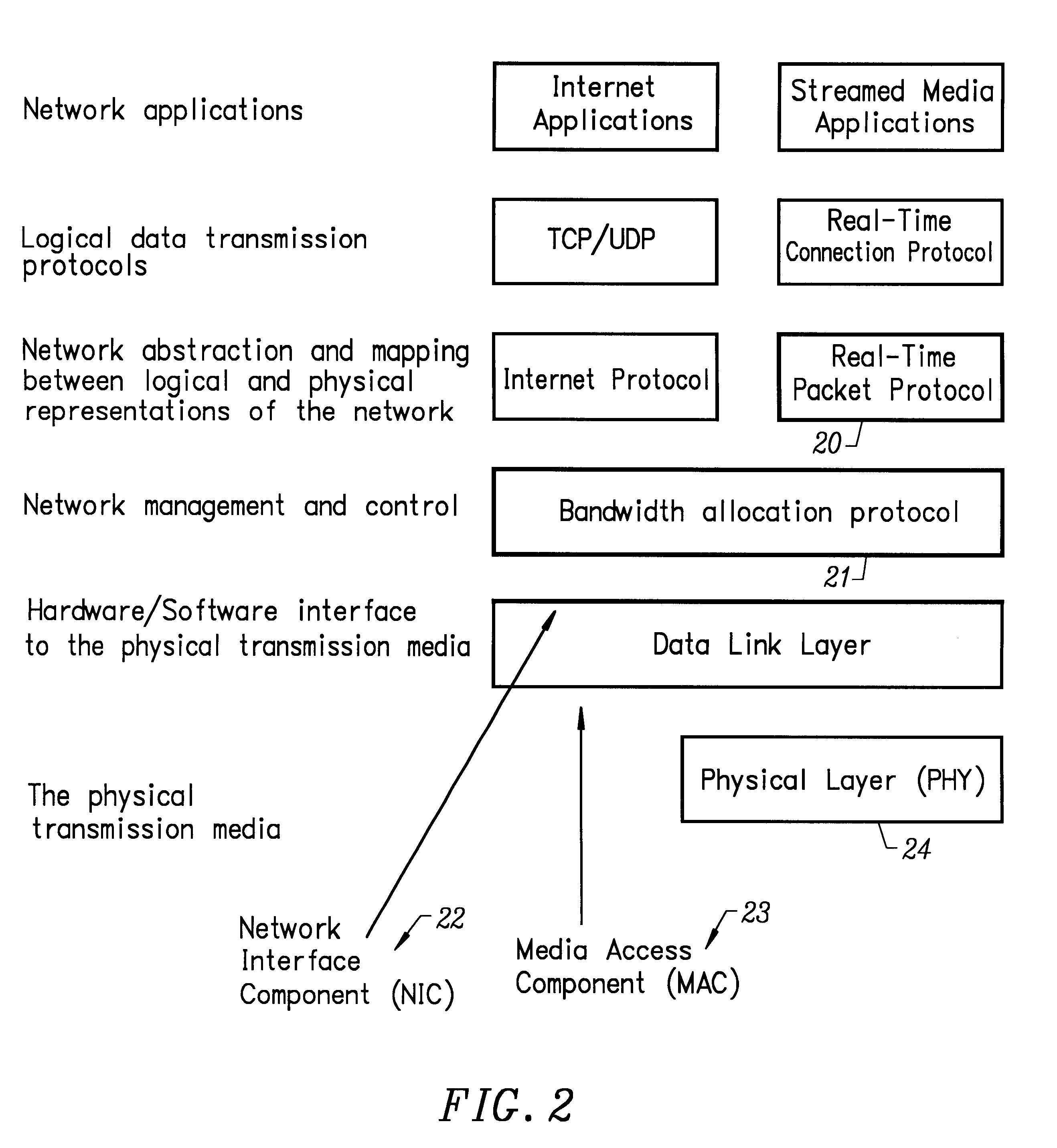
Method and apparatus implementing a multimedia digital network
InactiveUS6310886B1Most efficientHybrid switching systemsTime-division multiplexData capacityLong term data
A method and apparatus for efficiently managing the allocation of available data capacity on a physically shared digital network among devices connected to that network is disclosed. Also disclosed is a method and apparatus for managing the ongoing timely movement of data on the shared network such that precise long-term data rates are achieved between attached devices with minimal additional buffering. The invention further comprises a method and apparatus which allows the use of any remaining network capacity for non time-critical data movement without the need for centralized access management.
Owner:TIVO SOLUTIONS INC
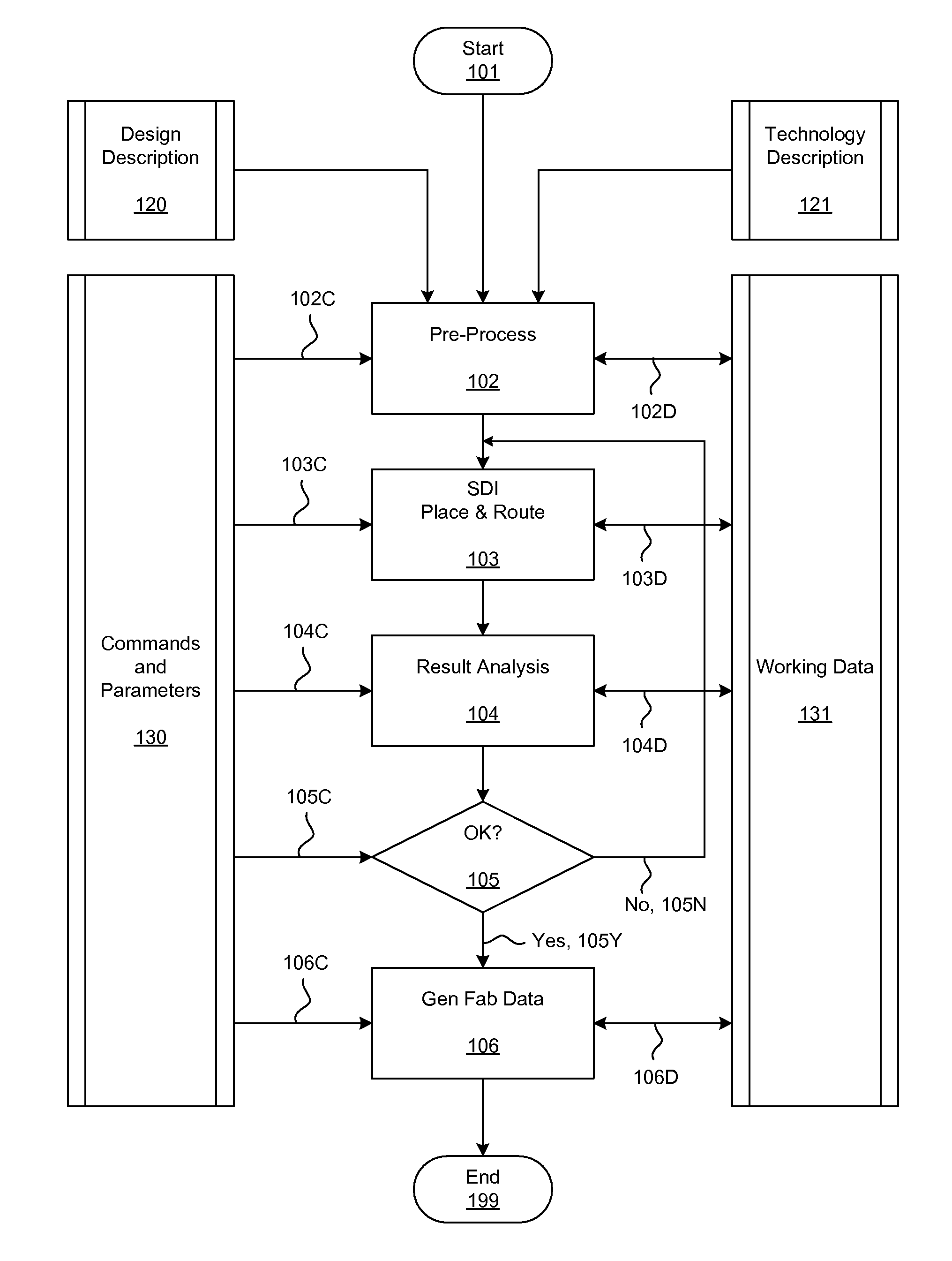
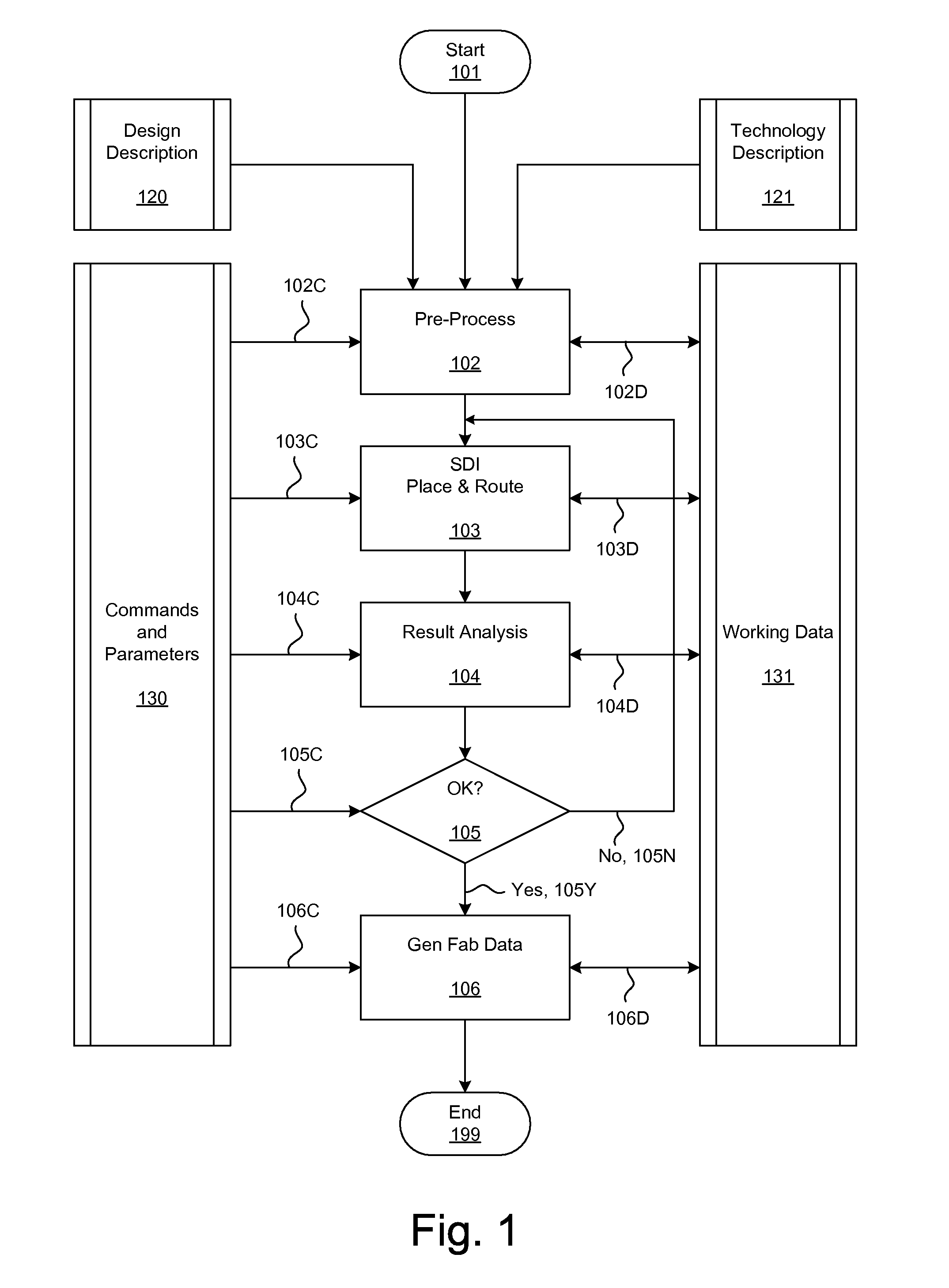
Methods and systems for placement and routing
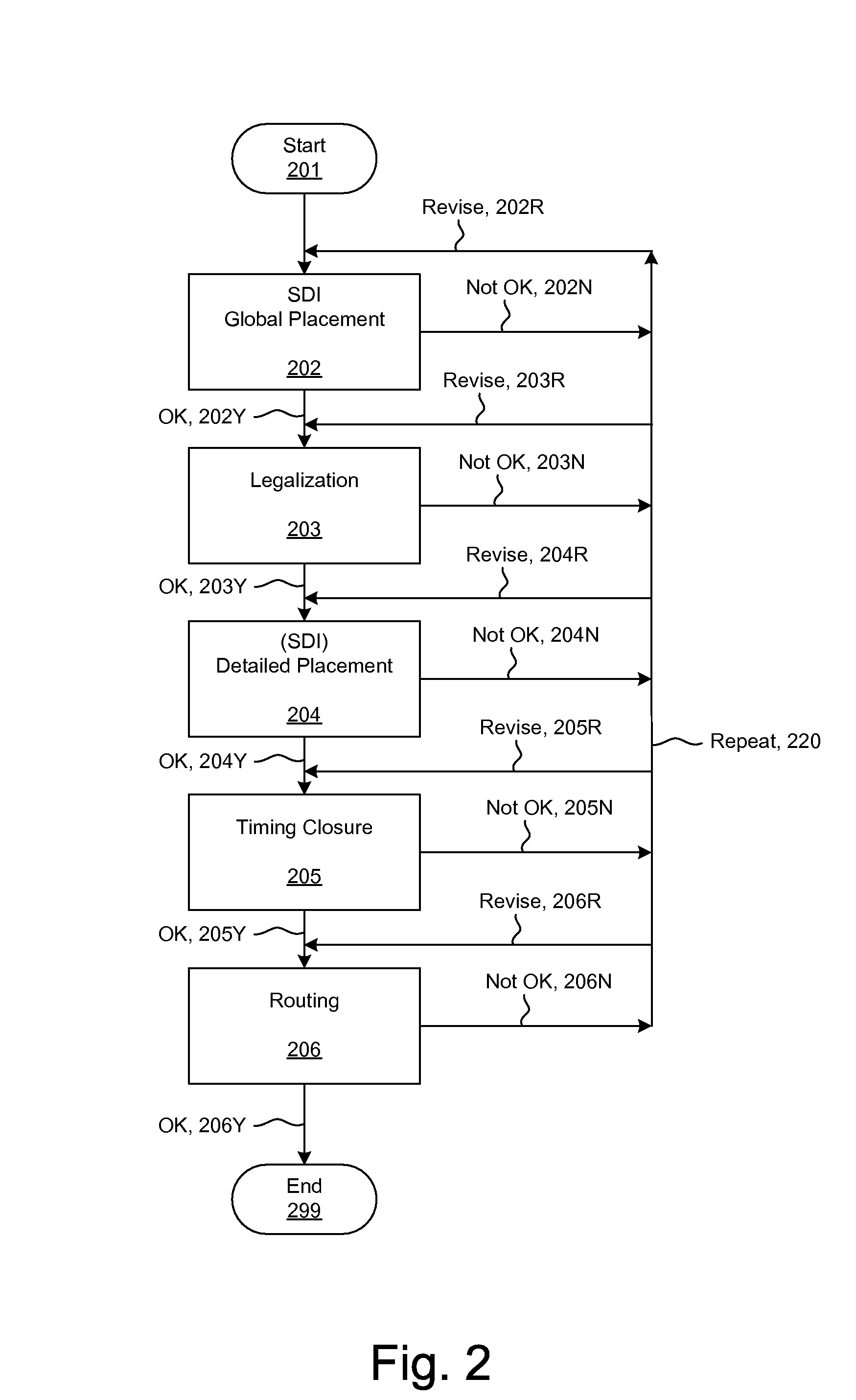
ActiveUS20090254874A1Influence optimizeImprove performanceCAD circuit designSoftware simulation/interpretation/emulationElectrical resistance and conductanceCapacitance
Techniques for placement of integrated circuit elements include global placement, detailed placement, timing closure, and routing. The integrated circuit is described by a netlist specifying interconnections of morphable devices. The detailed placement uses, for example, Simultaneous Dynamical Integration, wherein the morphable-devices correspond to nodes influenced by forces, including timing forces. The timing forces are derived, for example, from a timing graph; path delay; slack; and drive resistance of the elements. The timing closure uses timing-driven buffering and timing-driven resizing to reduce maximum delay and / or transition time, and / or to fix hold time. Nets having high capacitance and / or fanout, and timing critical nets are preferentially processed. Timing-driven buffering applies buffering solutions to segments of route trees, combines solutions of adjoining segments, and prunes sets of solutions. Timing-driven resizing morphably replaces selected elements with upsized versions thereof.
Owner:CALLAHAN CELLULAR L L C
Non-volatile memory and method with phased program failure handling
InactiveUS20050166087A1Improve efficiencyReduce overheadMemory architecture accessing/allocationMemory adressing/allocation/relocationError processingTime critical
In a memory with block management system, program failure in a block during a time-critical memory operation is handled by continuing the programming operation in a breakout block. Later, at a less critical time, the data recorded in the failed block prior to the interruption is transferred to another block, which could also be the breakout block. The failed block can then be discarded. In this way, when a defective block is encountered during programming, it can be handled without loss of data and without exceeding a specified time limit by having to transfer the stored data in the defective block on the spot. This error handling is especially critical for a garbage collection operation so that the entire operation need not be repeated on a fresh block during a critical time. Subsequently, at an opportune time, the data from the defective block can be salvaged by relocation to another block.
Owner:SANDISK TECH LLC
Method and apparatus for effecting a handoff between two IP connections for time critical communications
InactiveUS6850503B2Error preventionFrequency-division multiplex detailsTelecommunicationsCommunication unit
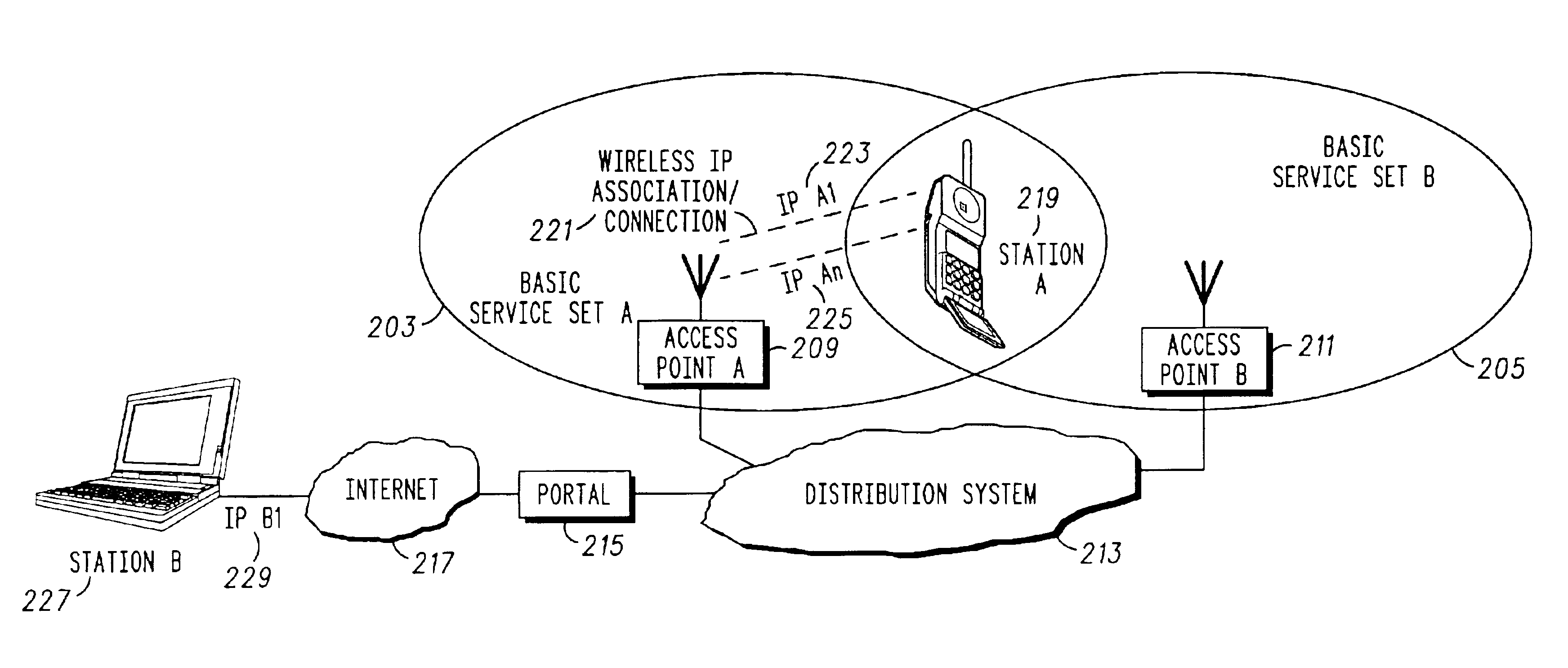
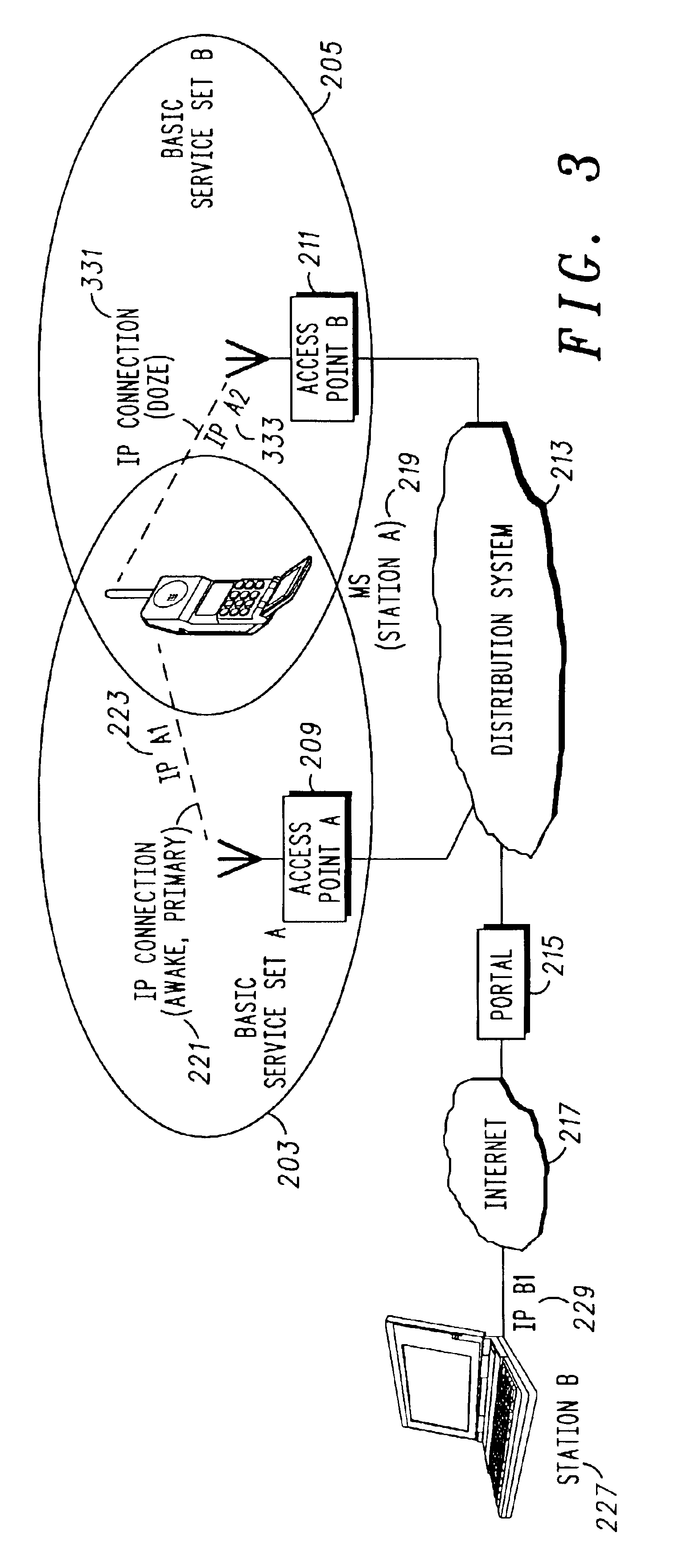
A method 900 of and wireless communications unit 800 for effecting a handoff from a first Internet Protocol (IP) connection 221 to a second IP connection 331 for a time critical communication is disclosed. The method includes communicating 905 between a first wireless station 219 and a second station 331 using the first IP connection and a first IP address 223 for the first wireless station; setting up 907 the second IP connection with a second IP address for the first wireless station, the first IP connection being a primary connection and the second IP connection being a secondary connection, both existing concurrently; determining 915 that the second IP connection should be the primary connection; and changing 917 the second IP connection to the primary connection by informing the second station that the second IP address is the primary address using stream control transmission protocol (SCTP) messages, wherein the time critical communication is immediately switched over to the second IP connection.
Owner:GOOGLE TECH HLDG LLC
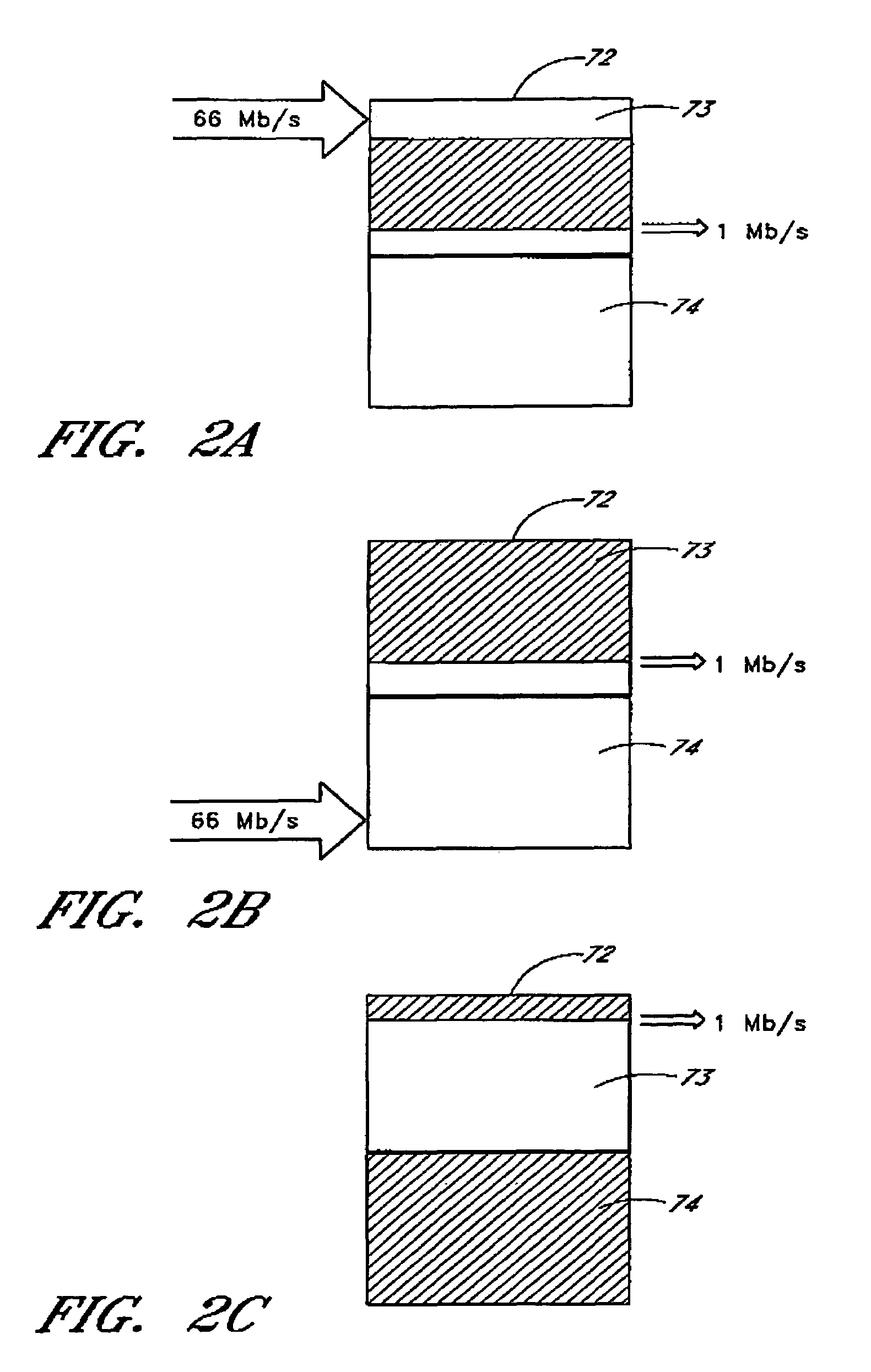
Video recording system utilizing storage redundancy to transfer non-time-critical, error-intolerant data segments while transferring time-critical, error-tolerant streaming data segments at a required data transfer rate
InactiveUS7133600B1Maintaining data transfer bandwidthEasy transferTelevision system detailsRecording carrier detailsStreaming dataData segment
The present invention may be regarded as a video recording system and method of transferring a non-time-critical, error-intolerant data segment stored on a disk drive, which is responsive to a set of data transfer commands generated by a host processor and which is operating in a mode optimized for transferring time-critical, error-tolerant streaming data segments stored or to be stored on the disk drive. The method includes sending a sequence of data transfer commands generated by the host processor to the disk drive to transfer a respective sequence of time-critical, error-tolerant streaming data segments at a required data transfer rate. The method further includes selectively interposing a first data transfer command into the sequence of data transfer commands, the first data transfer command initiating a first transfer of the non-time-critical, error-intolerant data segment from a first storage location. The method further includes transmitting a data transfer error signal generated by the disk drive to the host processor, the data transfer error signal having a state that indicates whether any data transfer errors have occurred with respect to the first transfer of the non-time-critical, error-intolerant data segment. The method further includes selectively interposing a second data transfer command into the sequence of data transfer commands, the second data transfer command initiating a second transfer of the non-time-critical, error-intolerant data segment from a second storage location, thereby utilizing storage redundancy to achieve an accuracy required for the non-time-critical, error-intolerant data segment while maintaining the required data transfer rate of the sequence of time-critical, error-tolerant data segments.
Owner:KEEN PERSONAL MEDIA +1
Methods and apparatus for modularization of real time and task oriented features in wireless communications
A wireless telephone comprising a basic telephone module adapted to perform time critical functions, and an enhanced services module adapted to perform non time critical functions is disclosed. The basic telephone module includes processing resources needed to provide communication, while the enhanced services module performs enhanced functions which can be performed on a non time critical basis so that processing resources belonging to the basic telephone module do not need to be diverted to these functions but instead can be dedicated to performing time critical functions. The basic telephone module can be removed from the enhanced services module and connected to a different enhanced services module, similarly, the enhanced services module can be removed from the basic telephone module and connected to a different basic telephone module. This feature allows a user to retain his or her basic telephone module or enhanced services module when acquiring a new or upgraded enhanced services module or basic telephone module.
Owner:LUCENT TECH INC
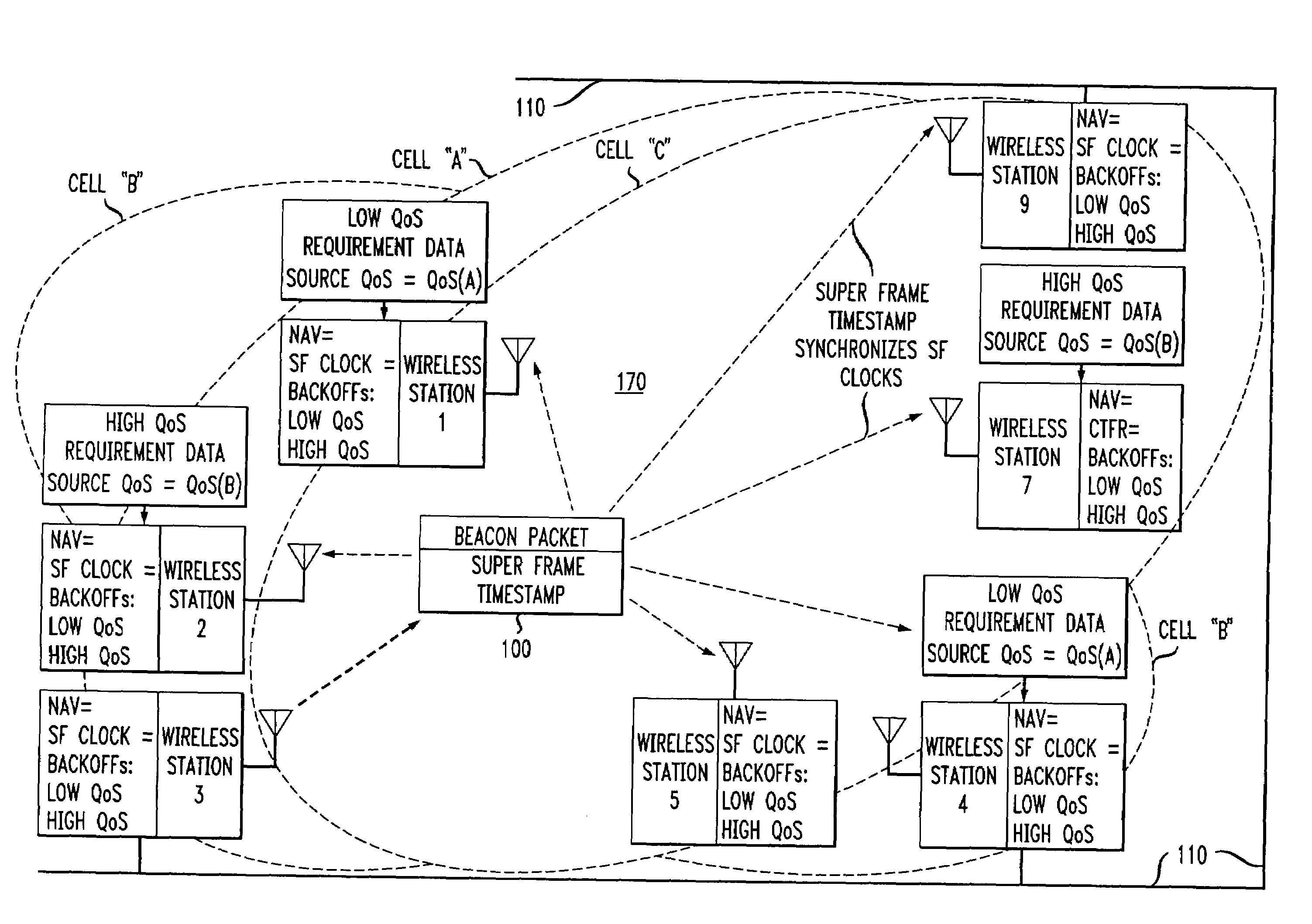
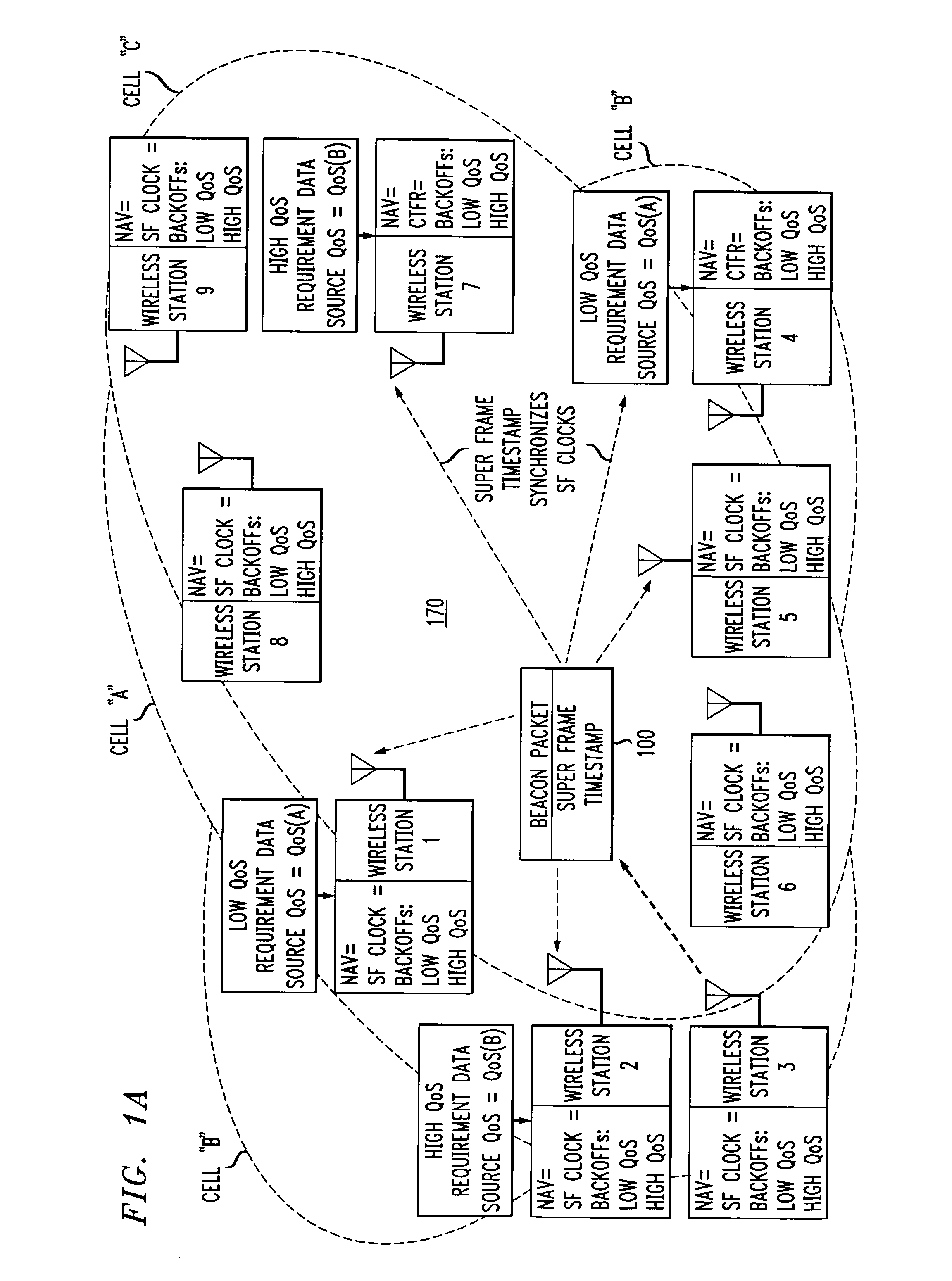
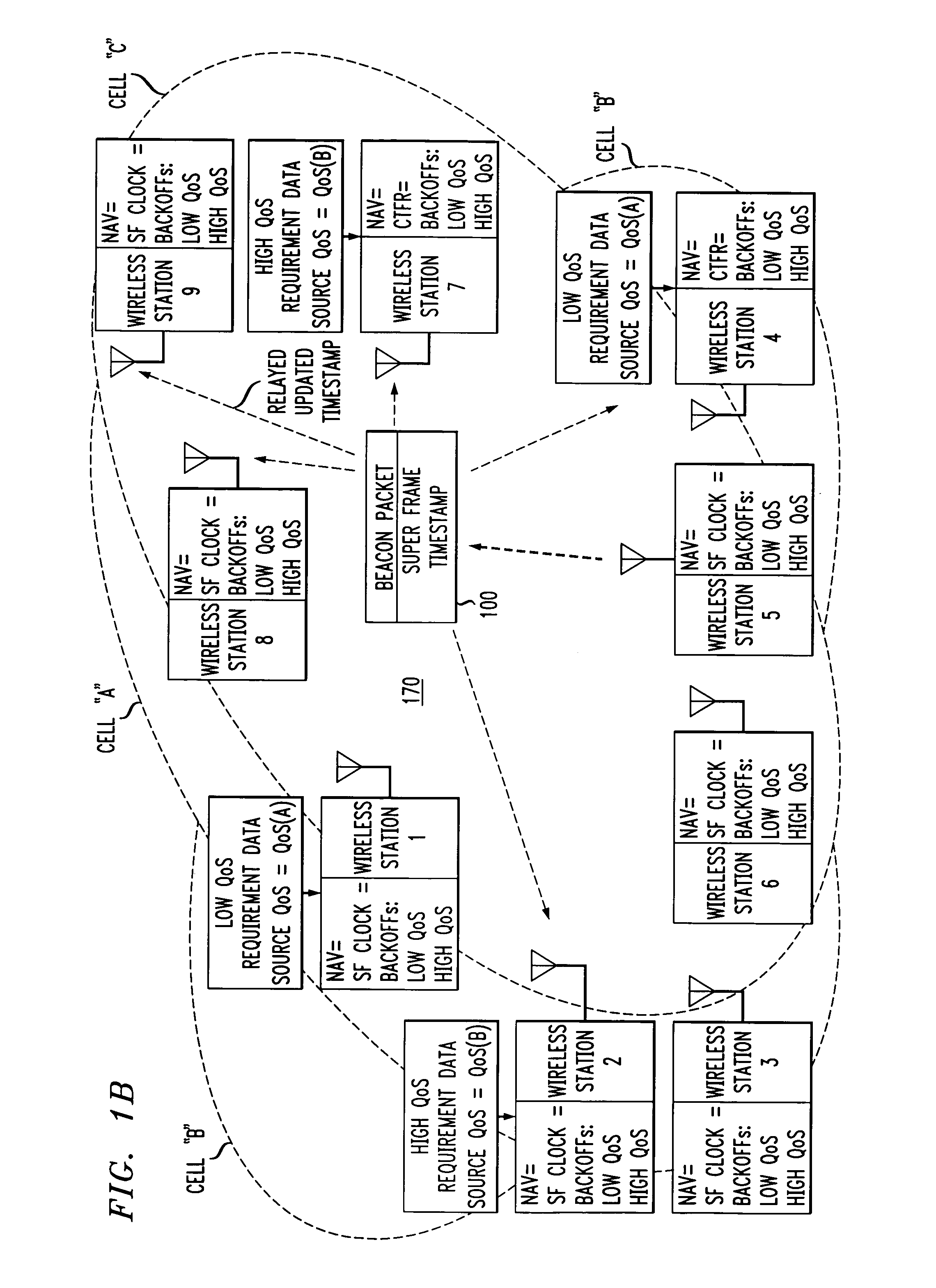
Wireless LANs and neighborhood capture
ActiveUS7280517B2Eliminates unfairnessReduce decreaseSynchronisation arrangementNetwork topologiesTimestampAccess method
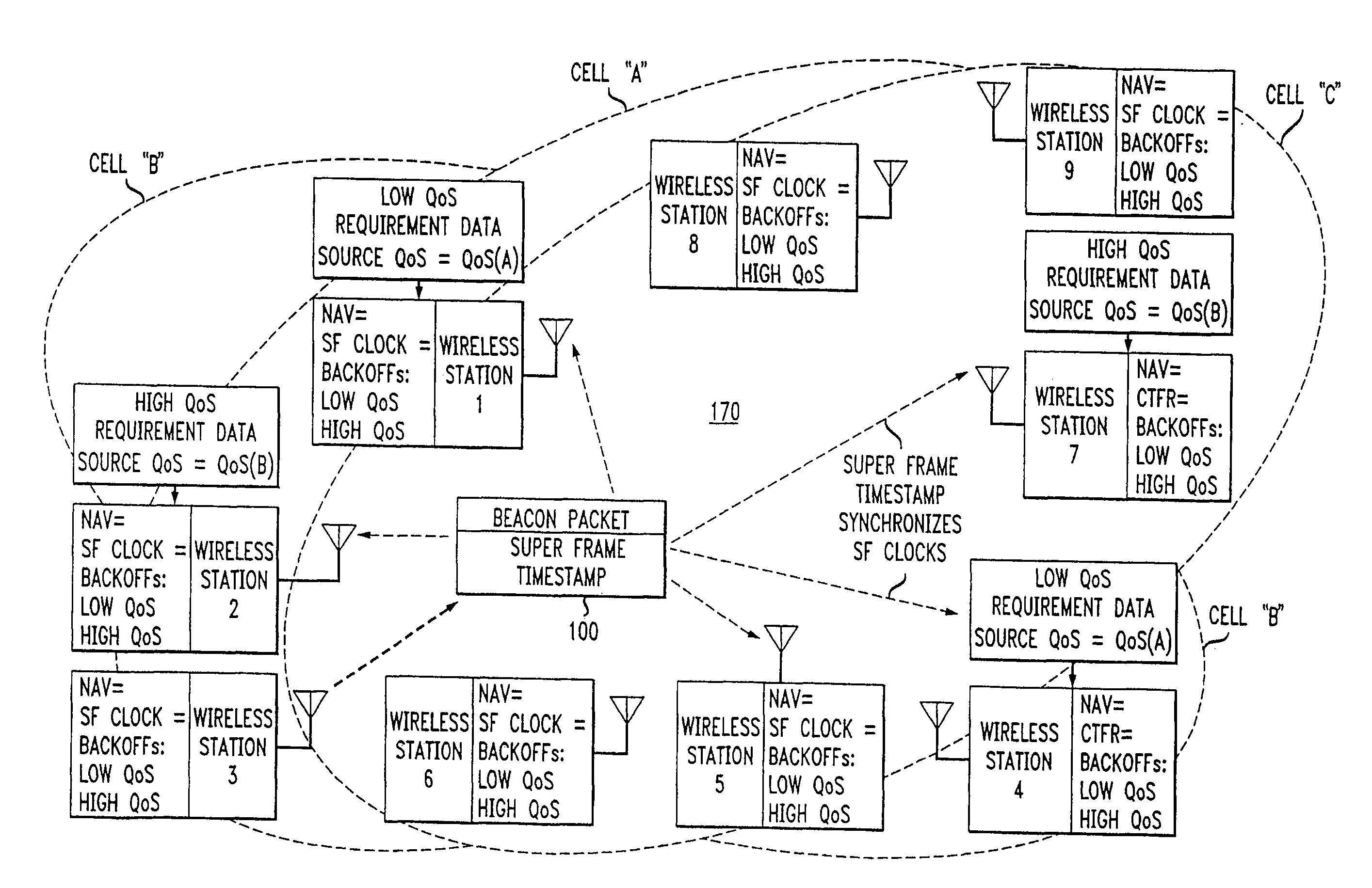
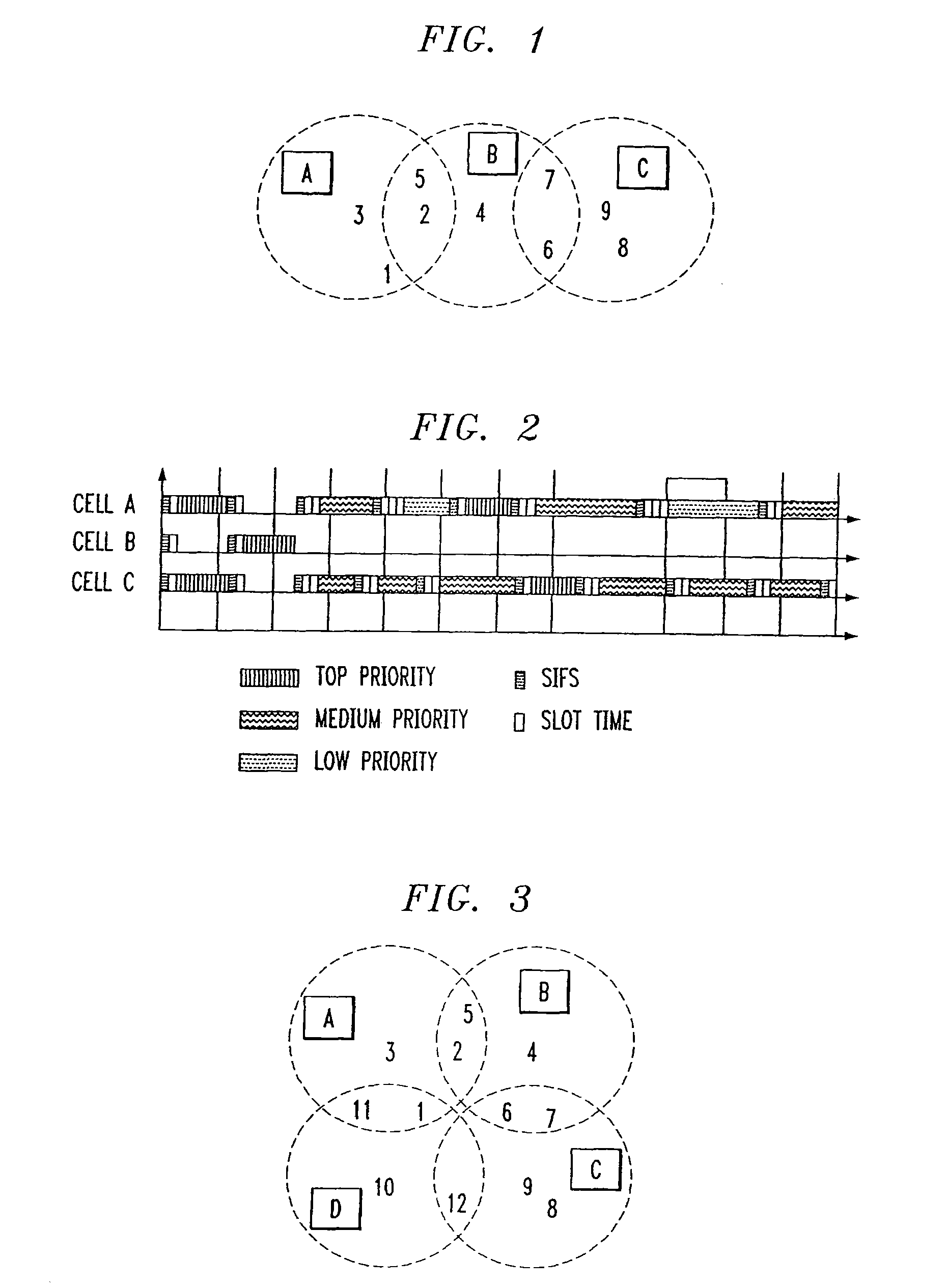
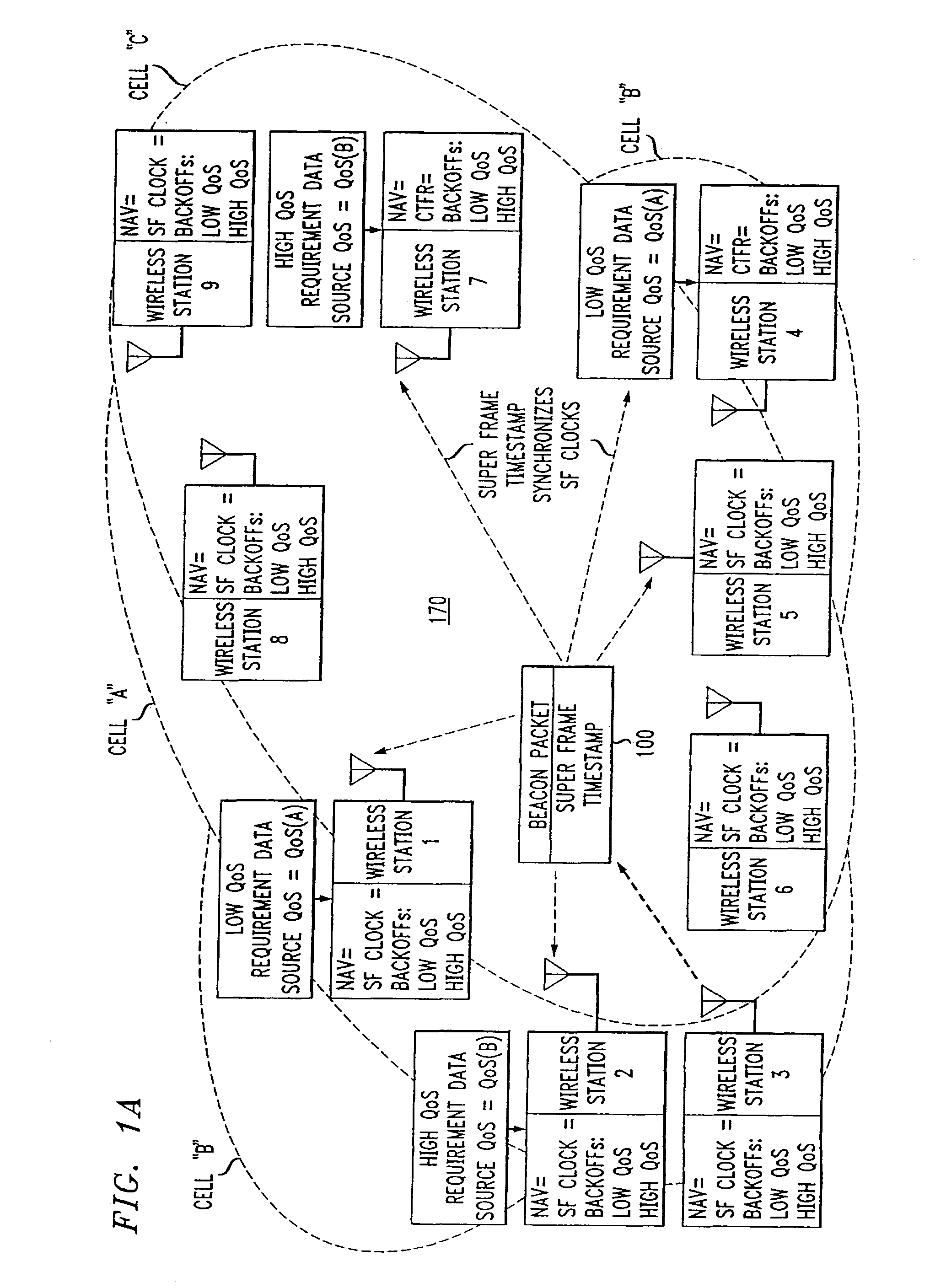
Overlapped wireless LAN cells in a medium have an equal chance at establishing a session on the medium. A first member station in the first cell transmits a timing packet containing a timestamp value, which is received at a second member station in the second cell. This synchronizes member stations in the first and second cells to interrupt transmissions at a global channel release instant corresponding to the timestamp value. The member stations in the first and second cells then have the opportunity to contend for access to the medium following the global channel release instant, using a slotted CSMA / CA access method. Each of the member stations in the first and second cells has a superframe clock that is synchronized based on the timestamp value, thereby establishing a periodic global channel release instant during each of a plurality of periodic superframes. The member stations can then periodically interrupt transmissions at the periodic global channel release instant to contend for the medium. The periodic global channel release instant occurs at intervals that are sufficiently close to meet delay and jitter restrictions for time-critical voice and video applications.
Owner:AT&T INTPROP I L P
Geo-cast systems and methods
ActiveUS20050096065A1Superior flexibility and scaling propertySpecial service provision for substationBroadcast service distributionTime criticalGeolocation
A system and method for communicating with only a subquantity of mobile receivers operating within a geographic area that the sender wishes to communicate with, particularly for time critical messages. A system for geo-casting messages to at least one receiver within a geographic region is provided. The system includes an input for receiving the message and a circuit coupled to the input. Upon receiving the message, the circuit reads a geographic designator. Then the circuit accesses a geospatial database using the geographic designator whereby the circuit determines which receivers are in the geographic region designated by the geographic designator. From the geospatial database, the circuit also determines addresses for the receivers so that the circuit can individually forward the message to the receivers within the designated geographic region.
Owner:THE BOEING CO
Geo-cast systems and methods
ActiveUS7613467B2Superior flexibility and scaling propertySpecial service provision for substationBroadcast service distributionTime criticalComputer science
A system and method for communicating with only a subquantity of mobile receivers operating within a geographic area that the sender wishes to communicate with, particularly for time critical messages. A system for geo-casting messages to at least one receiver within a geographic region is provided. The system includes an input for receiving the message and a circuit coupled to the input. Upon receiving the message, the circuit reads a geographic designator. Then the circuit accesses a geospatial database using the geographic designator whereby the circuit determines which receivers are in the geographic region designated by the geographic designator. From the geospatial database, the circuit also determines addresses for the receivers so that the circuit can individually forward the message to the receivers within the designated geographic region.
Owner:THE BOEING CO
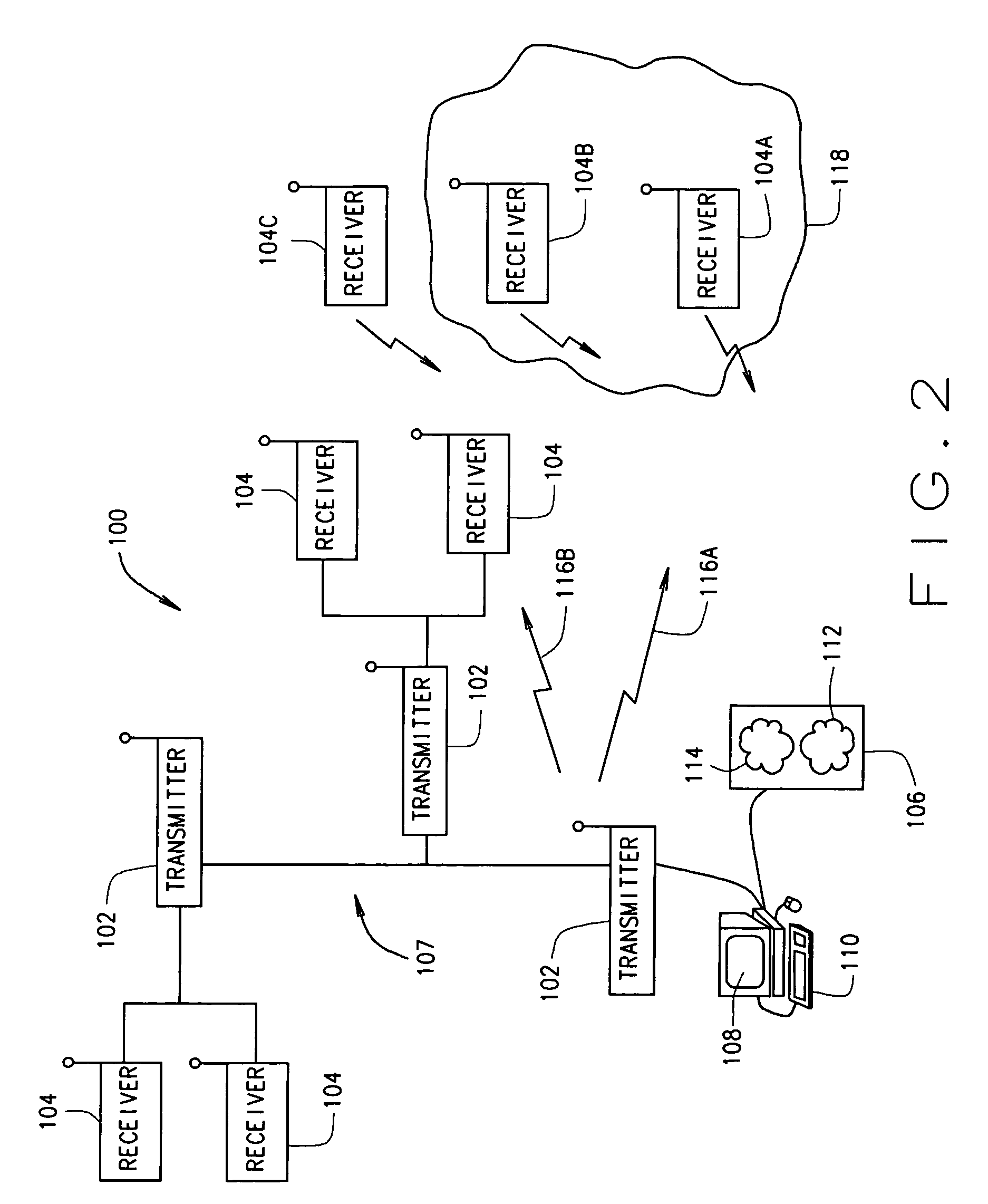
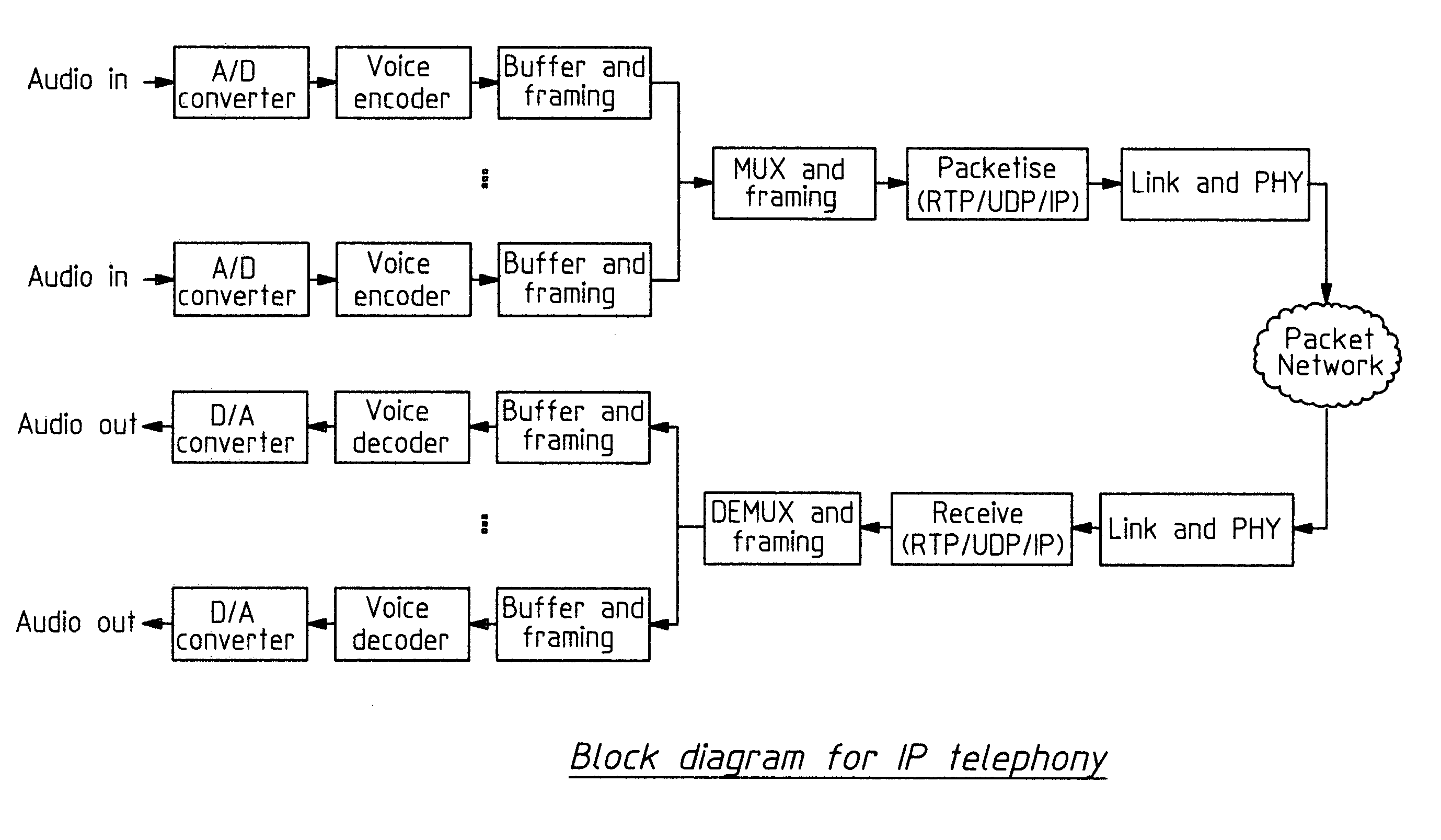
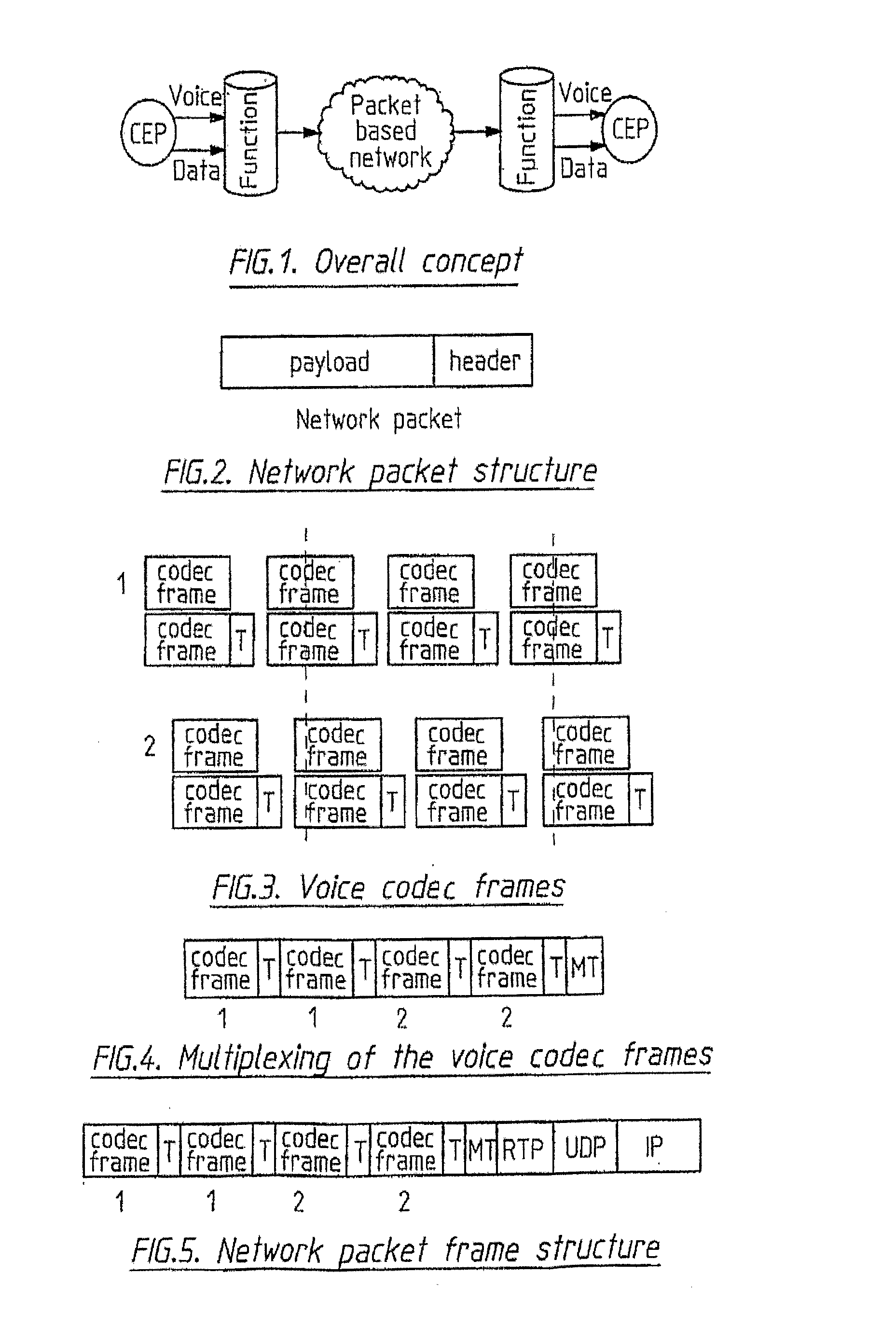
Packet based communications
InactiveUS20100014510A1Raise the ratioImprove efficiencyNetwork connectionsData trafficCommunication endpoint
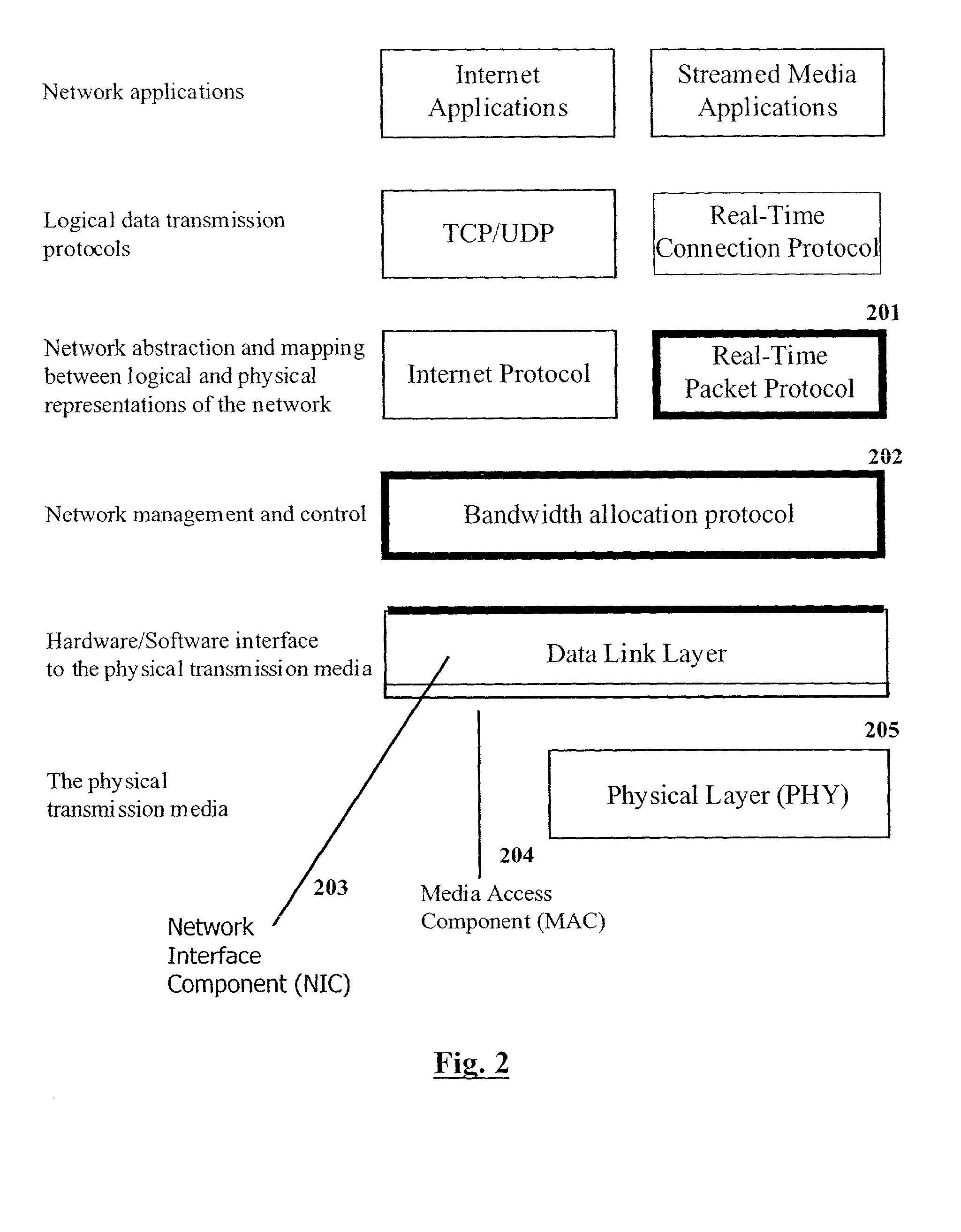
This invention is applicable to packet based, rate limited radio links, such as satellite or terrestrial wireless digital communications systems. These communications networks concurrently carry time-critical traffic, such as voice or multimedia, and non time-critical traffic, such as generic data traffic, between two or more communication end points. The communication end points may be connected through a number of heterogenous networks and the end to end throughput characteristics may vary over time. A first aspect of the invention concerns a method for generating packets. In other aspects the invention concerns a computer system for use in packet based communications, a computer protocol for packet based communications and a communications packet. The invention involves determining a “time slice” packet size from the link speed and the interval of time extending between the times at which packets are selected for output from a buffer to the transmission interface. It also involves creating a network packet from frames of time-critical data generated during the interval, where the packet is synchronised to both existing timing requirements of the time-critical frames and the link speed. Then, adding non time-critical data to the network packet if its size had not exceeded the determined “time slice” packet size.
Owner:NAT ICT AUSTRALIA
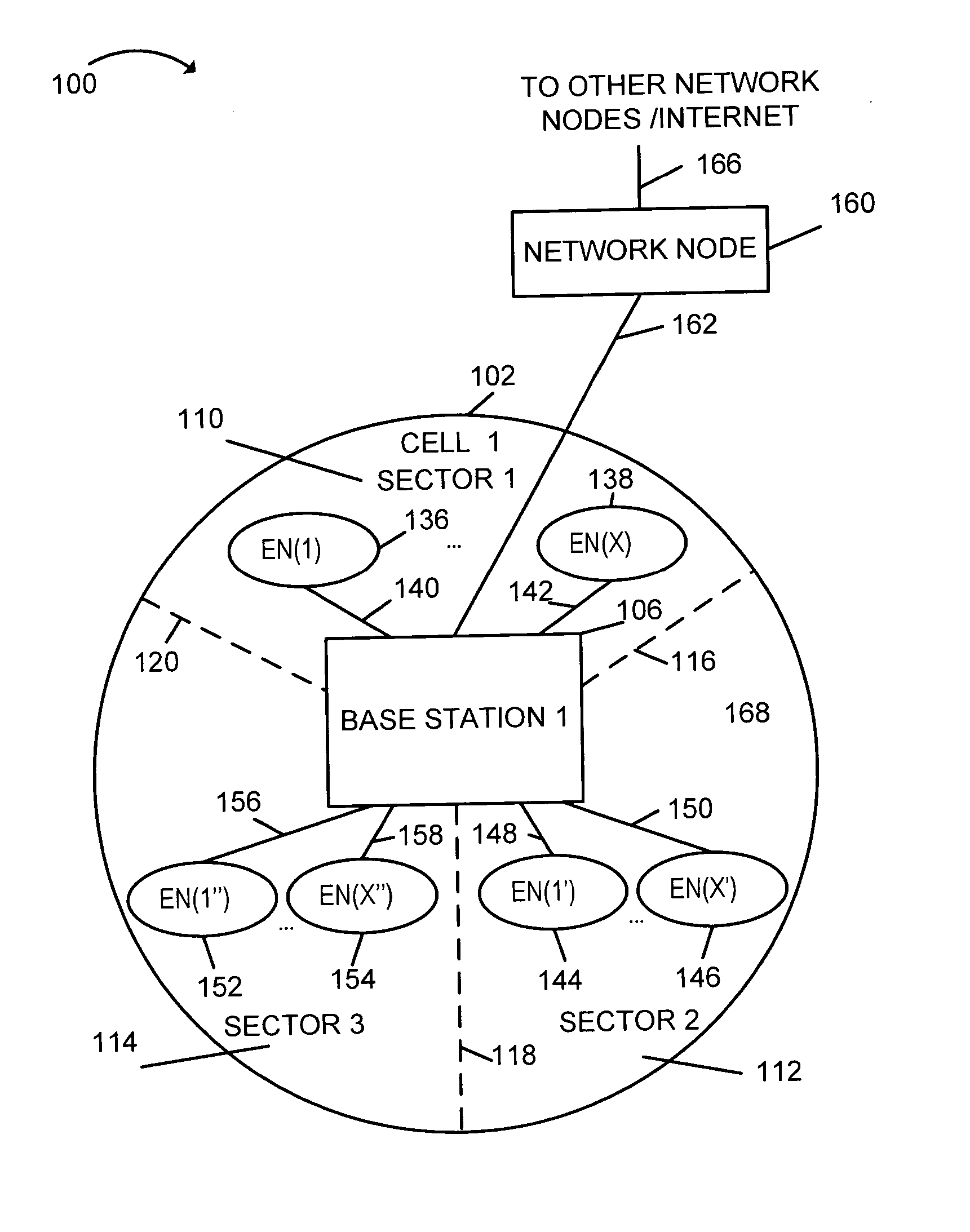
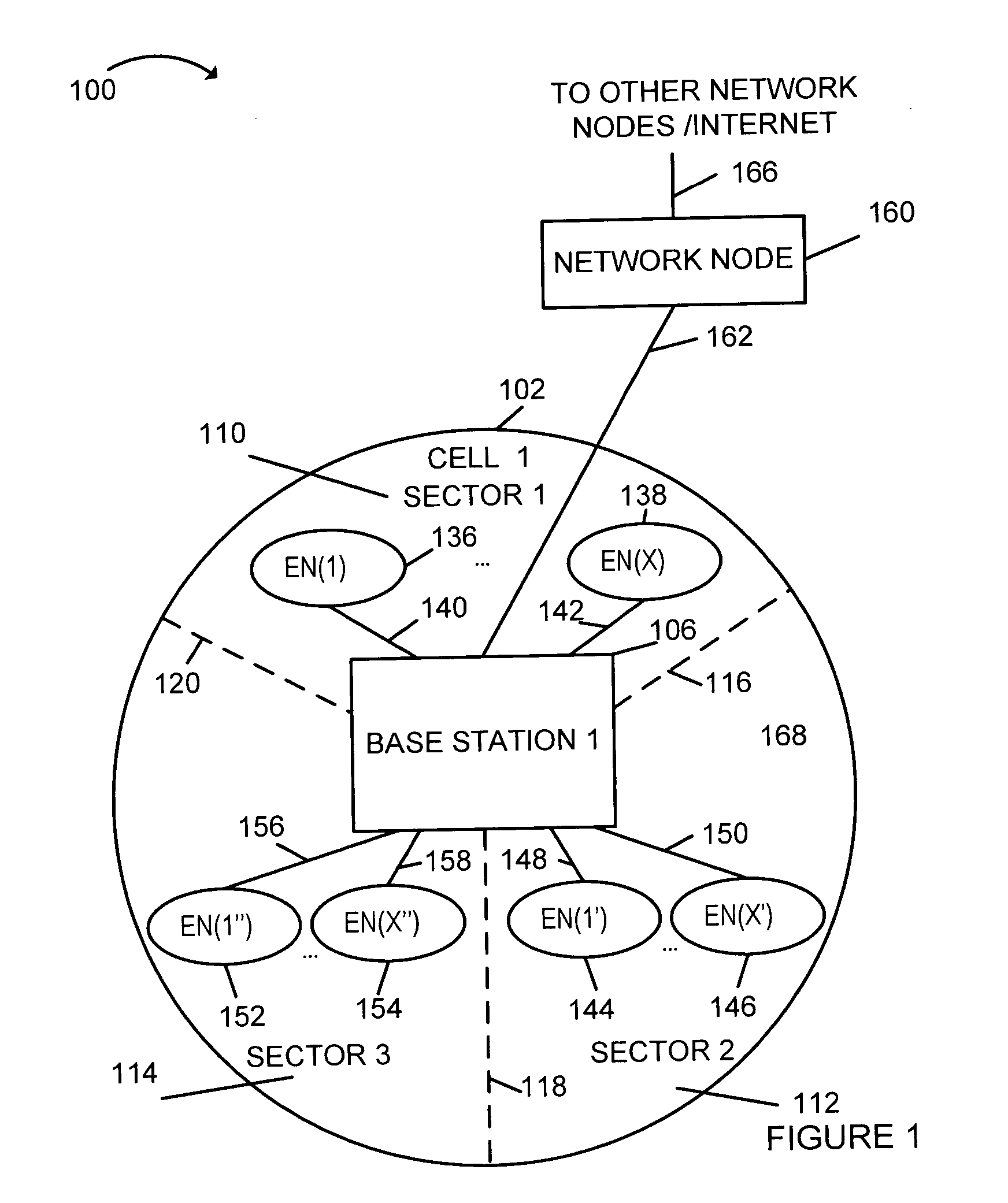
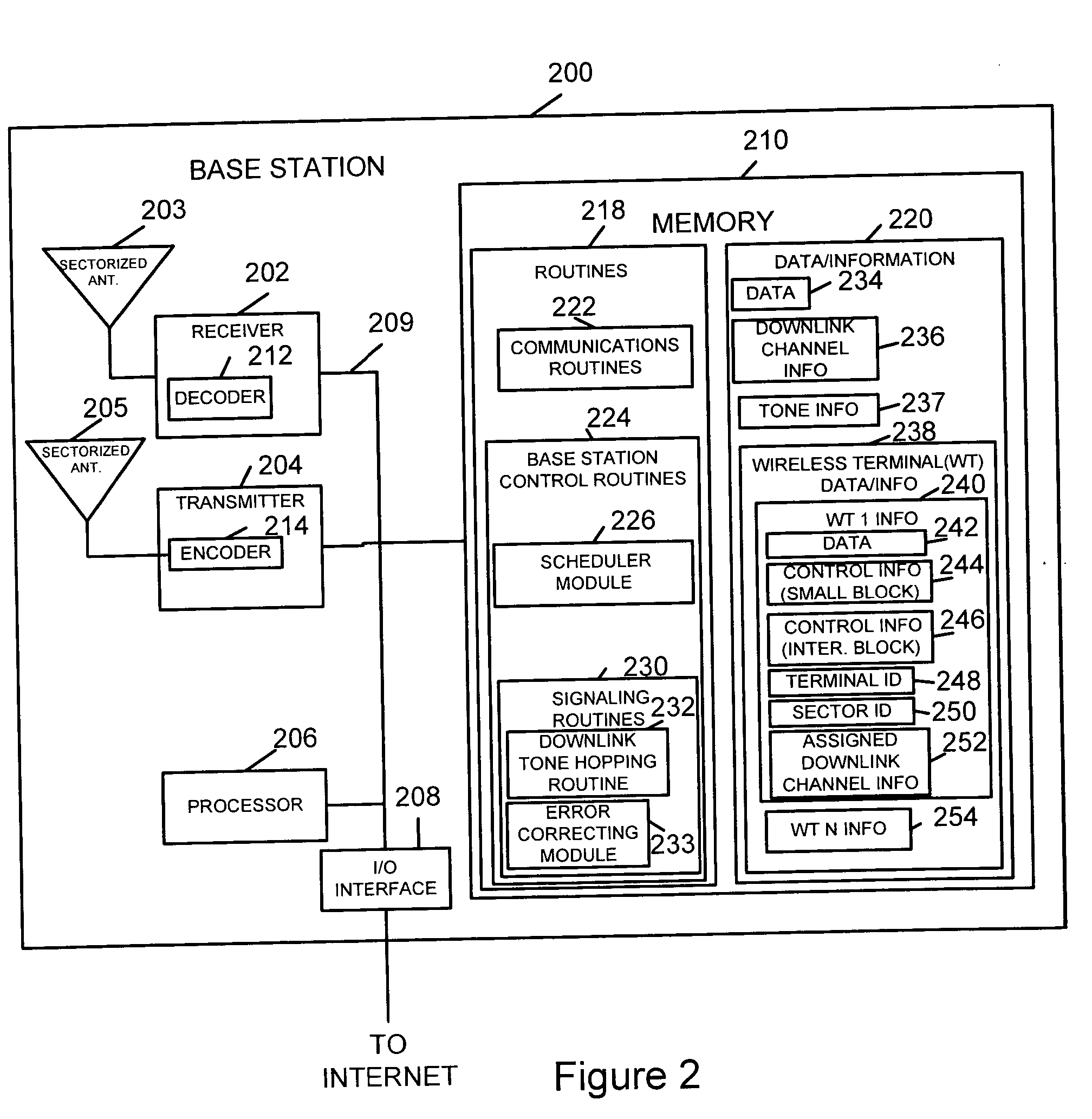
Method of downlink resource allocation in a sectorized environment
Methods and apparatus for communicating different size coded blocks of information in a wireless sectorized communications cell are described. Information may be categorized and formed into large, medium, and small coded blocks which may include error correction code bits based on the number of bits representing the information, time criticality of the information, and tolerable level of interference. Channels with full tone overlap between adjacent sectors, channels with no tone overlap between adjacent sectors, and channels with partial tone overlap between adjacent sectors are used for different size blocks. Some tones corresponding to a channel with less than full tone overlap are left unused in an adjacent sector thereby achieving less than full transmission tone overlap. Large transmission blocks are transmitted using full tone overlap channels; medium transmission blocks are transmitted using partial tone overlap channels; small transmission blocks are transmitted without using transmission tone overlap in adjacent sectors.
Owner:QUALCOMM INC
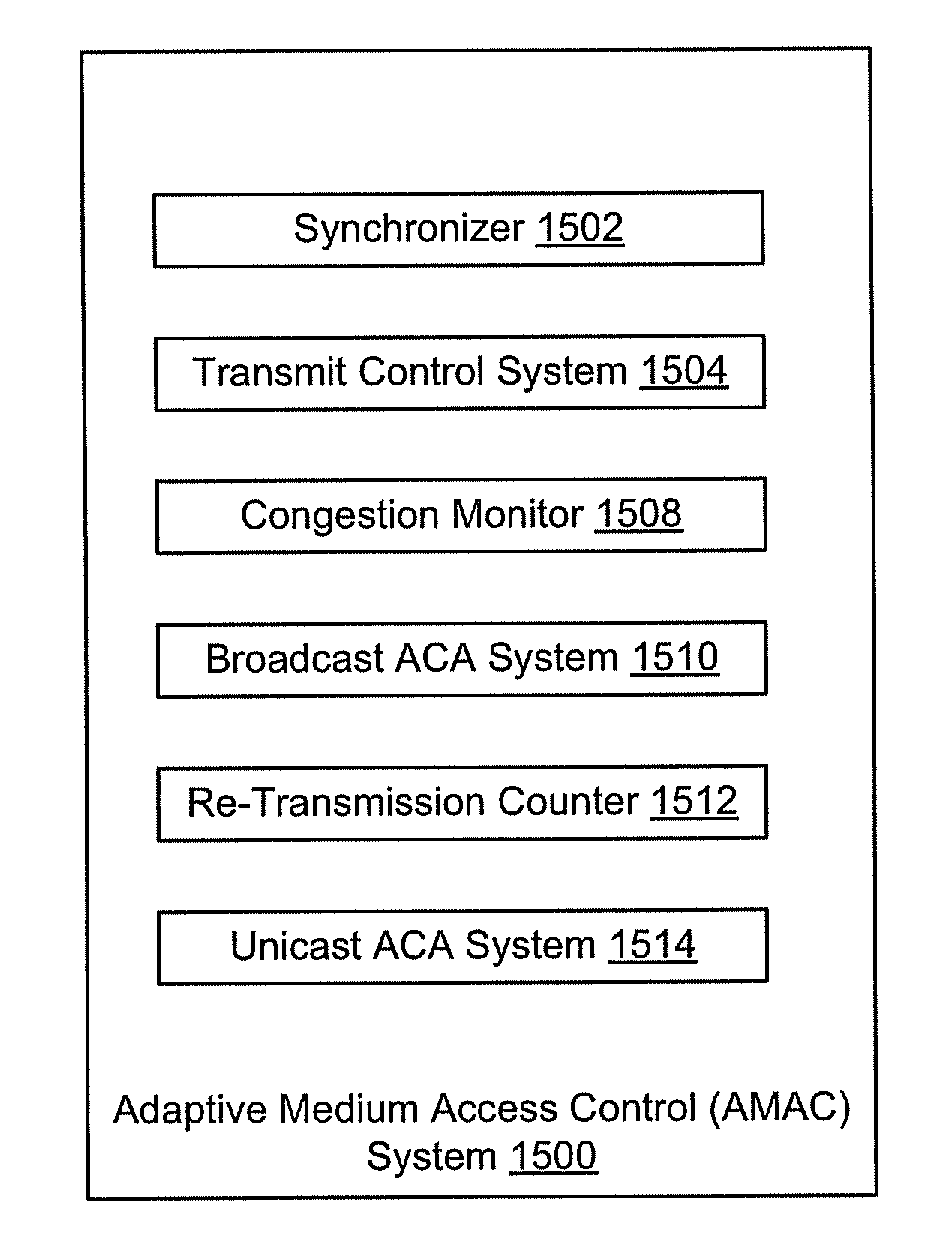
Adaptive Medium Access Control
ActiveUS20120182867A1Controlling the riskError preventionTransmission systemsPerformance enhancementTraffic flow
Bandwidth allocation configuration and fully decentralized adaptive medium access control (AMAC) systems and methods with support for time critical applications, spectrum efficiency, scalability enhancements, and fair allocation of bandwidth among nodes sharing a common channel. The methods fully integrate TDMA and CSMA / CA channel access approaches and incorporate adaptive congestion and collisions avoidance scheme to reduce bandwidth wastage and diminish adverse cross layers interactions. AMAC improves support for multi-media traffic while allowing higher transmission incidents from large number of transmitting devices sharing a common channel, with fair distribution of the available bandwidth, to enable improved multi-level-security connectivity over a common multi-hop wireless network, provide end-to-end performance enhancement for constant bit rate traffic, variable bit rate traffic, and distribute bandwidth fairly amongst competing TCP traffic flows that traverse varying length paths in multi-hop ad-hoc wireless networks.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
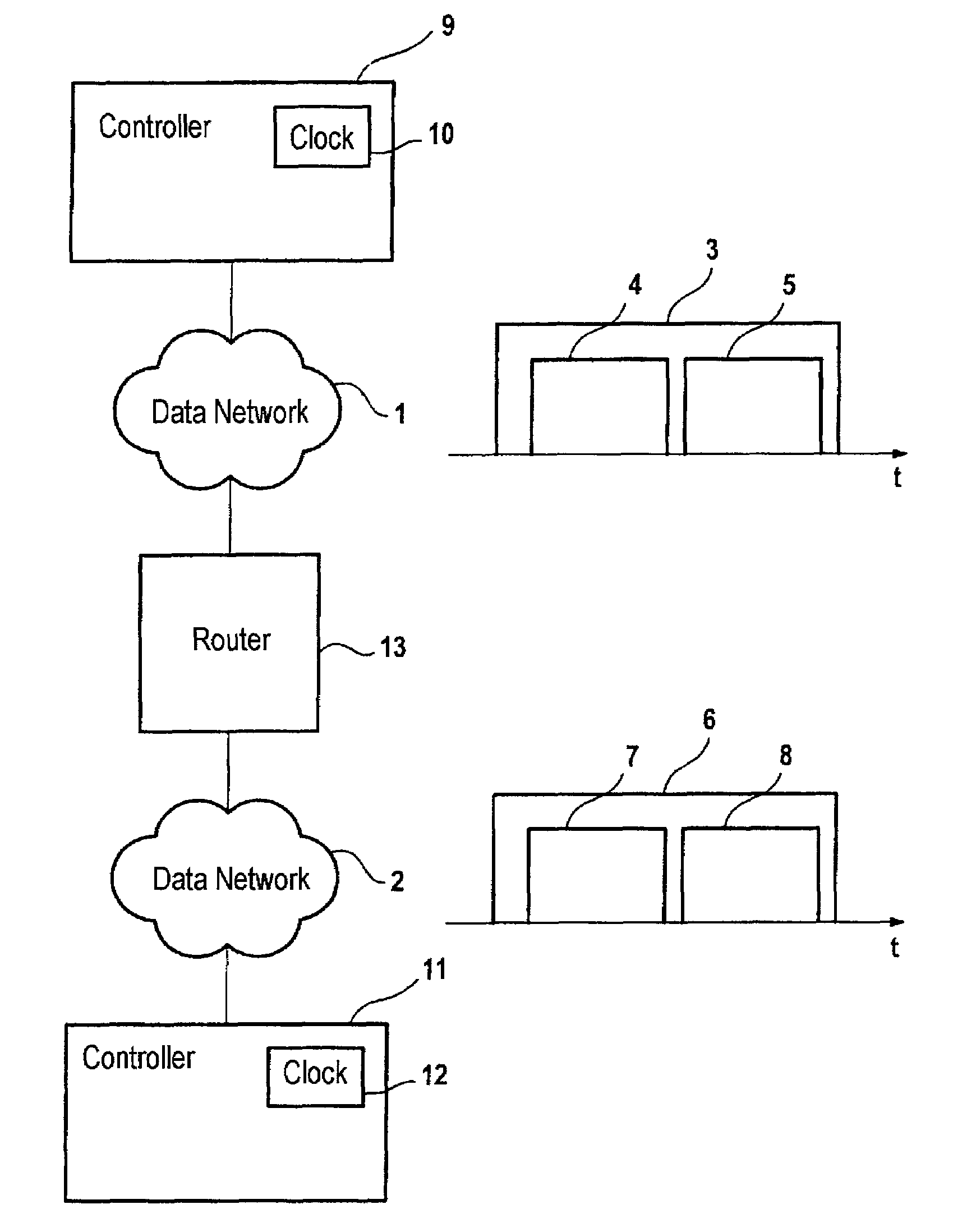
Method and system for coupling data networks
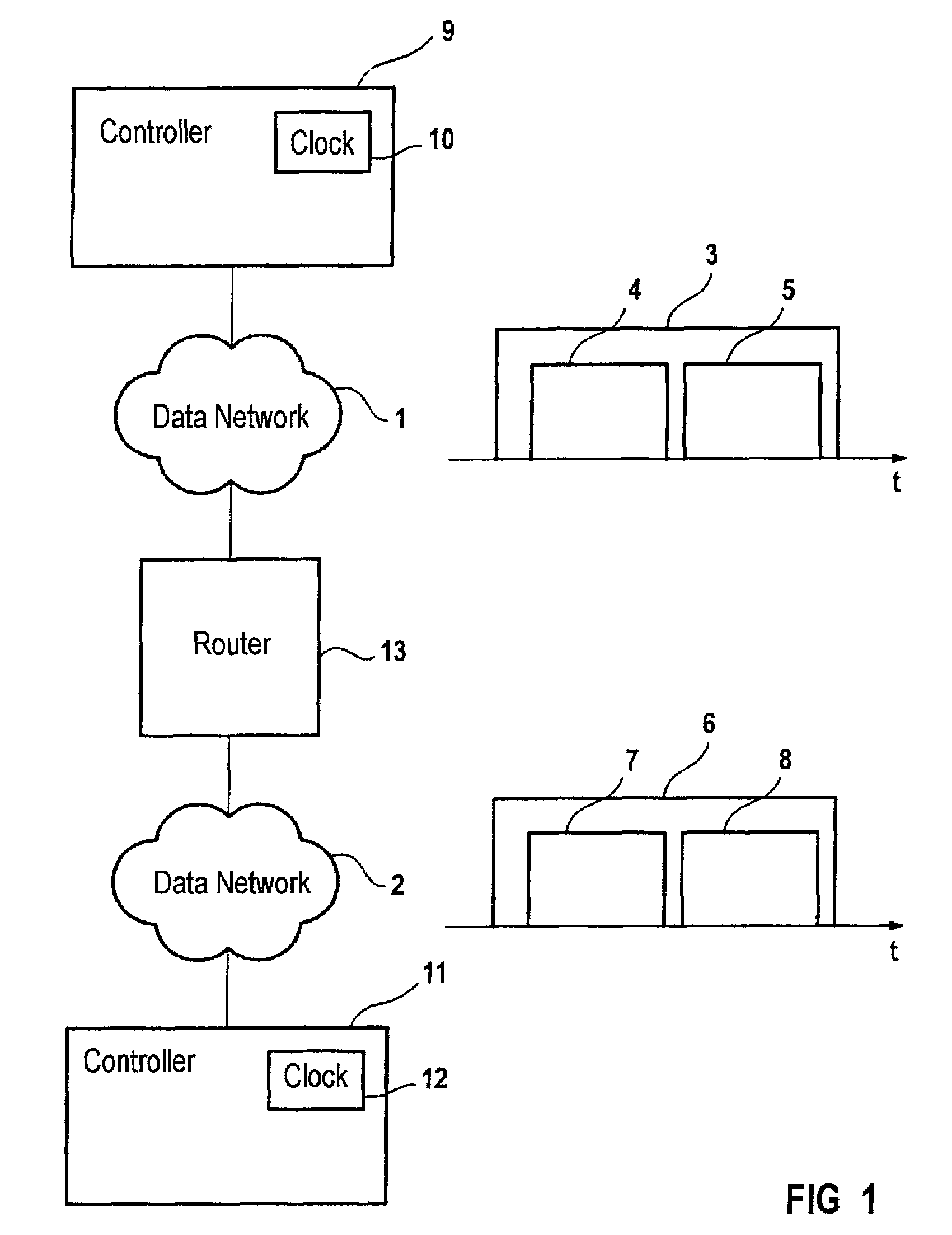
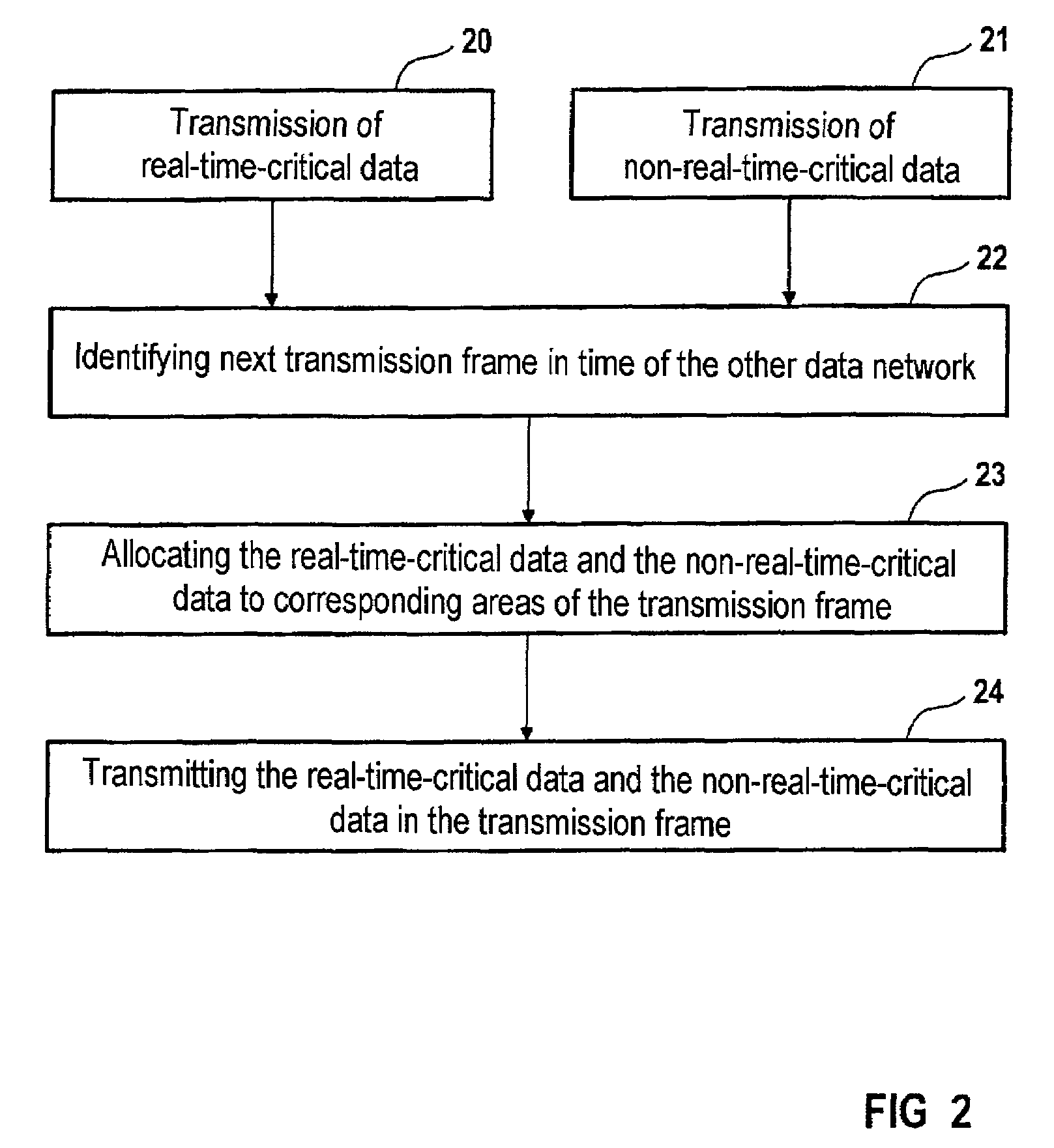
InactiveUS7411966B2Frequency-division multiplex detailsTime-division multiplexNon real timeTime critical
A method and a system for transmitting data by means of a first data network (1) have first means for transmitting data in at least one first transmission cycle, the first transmission cycle being subdivided into a first area (4) for transmitting real-time-critical data and a second area (5) for transmitting non-real-time-critical data, and by means of a second data network (2) having second means for transmitting data in at least one second transmission cycle, the second transmission cycle being subdivided into a third area (7) for transmitting real-time-critical data and into a fourth area (8) for transmitting non-real-time-critical data, and with a switching unit (13) for transmitting real-time-critical data of the first area into the third area.
Owner:SIEMENS AG
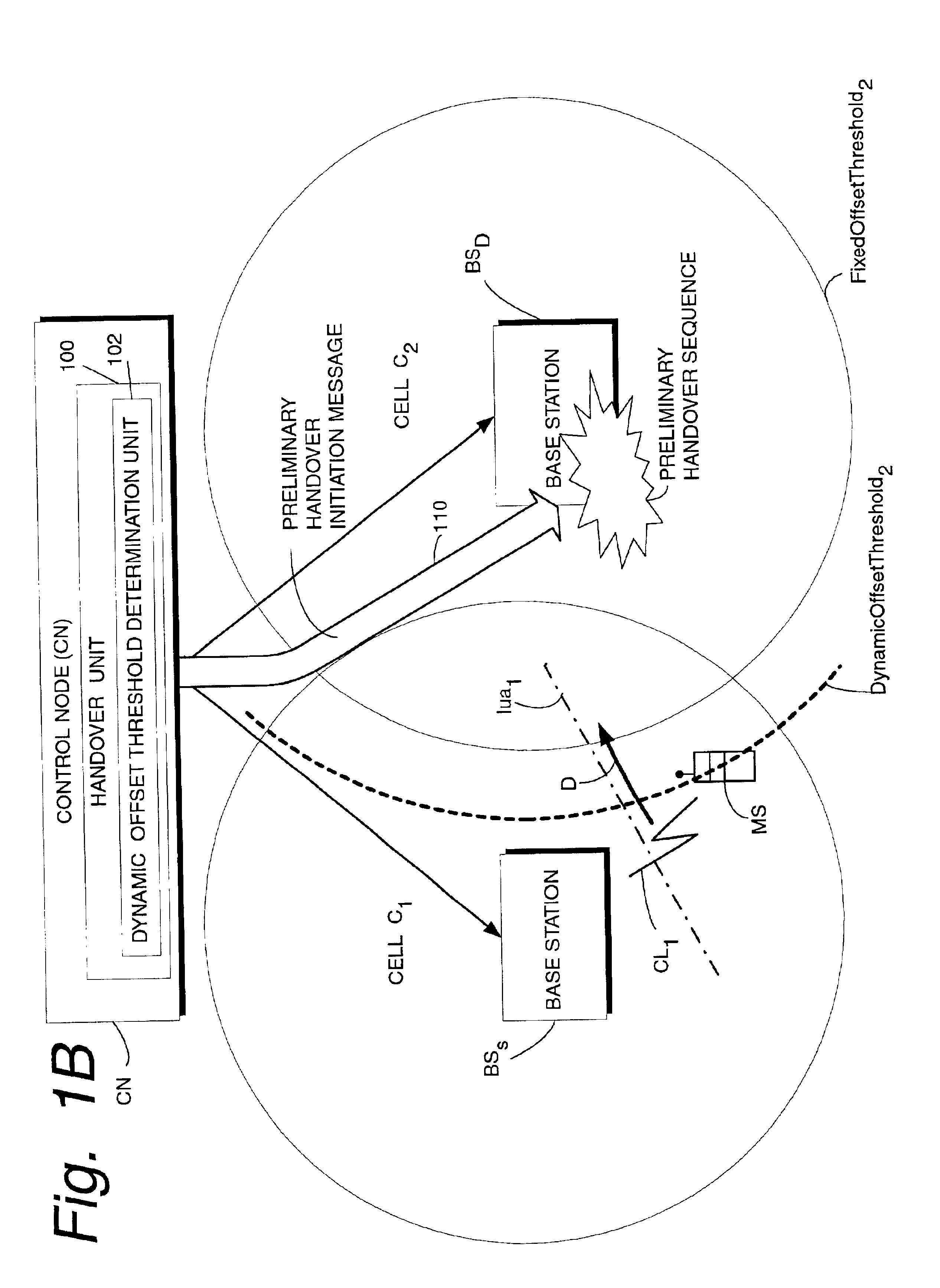
Dynamic offset threshold for diversity handover in telecommunications system
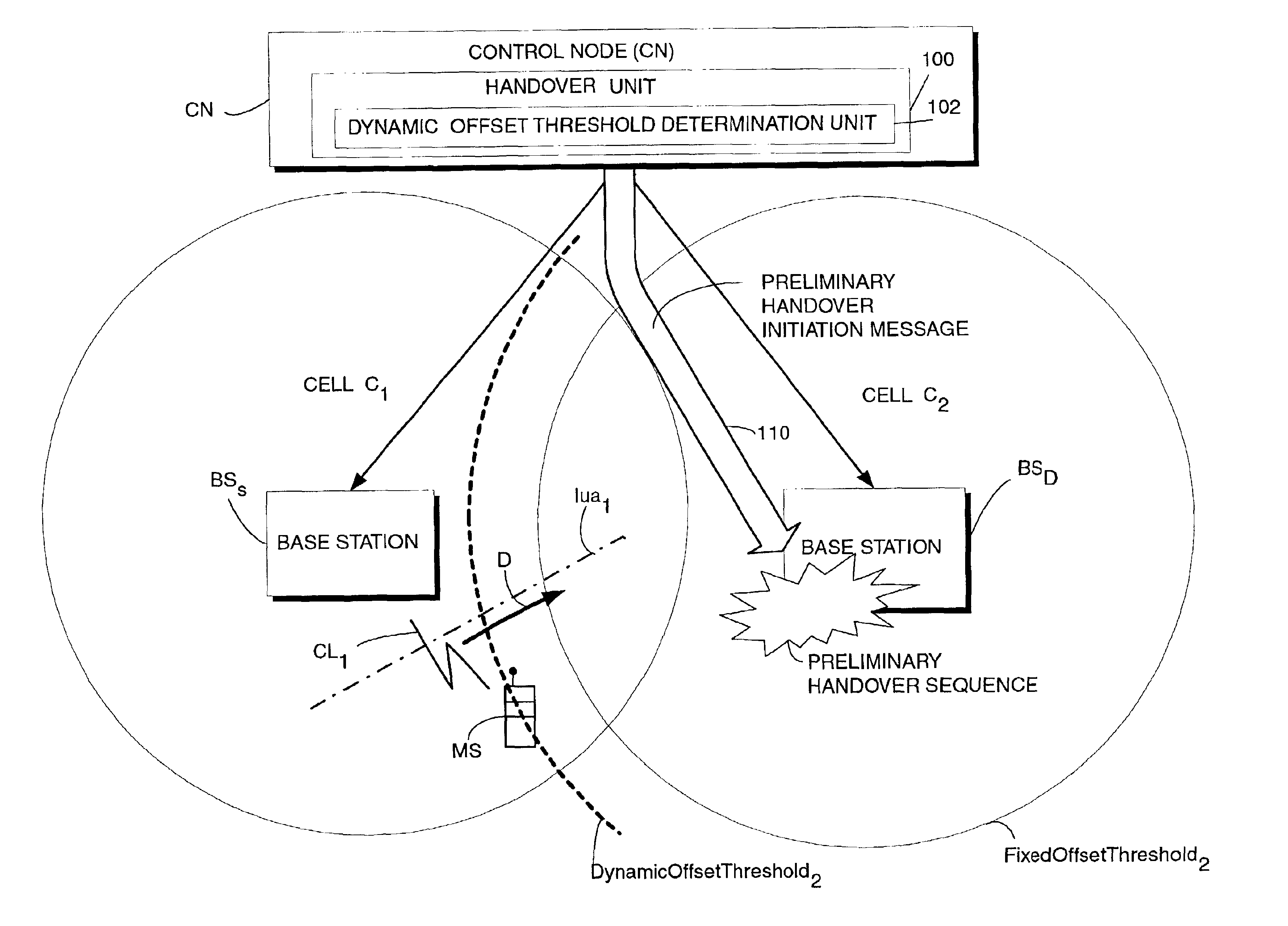
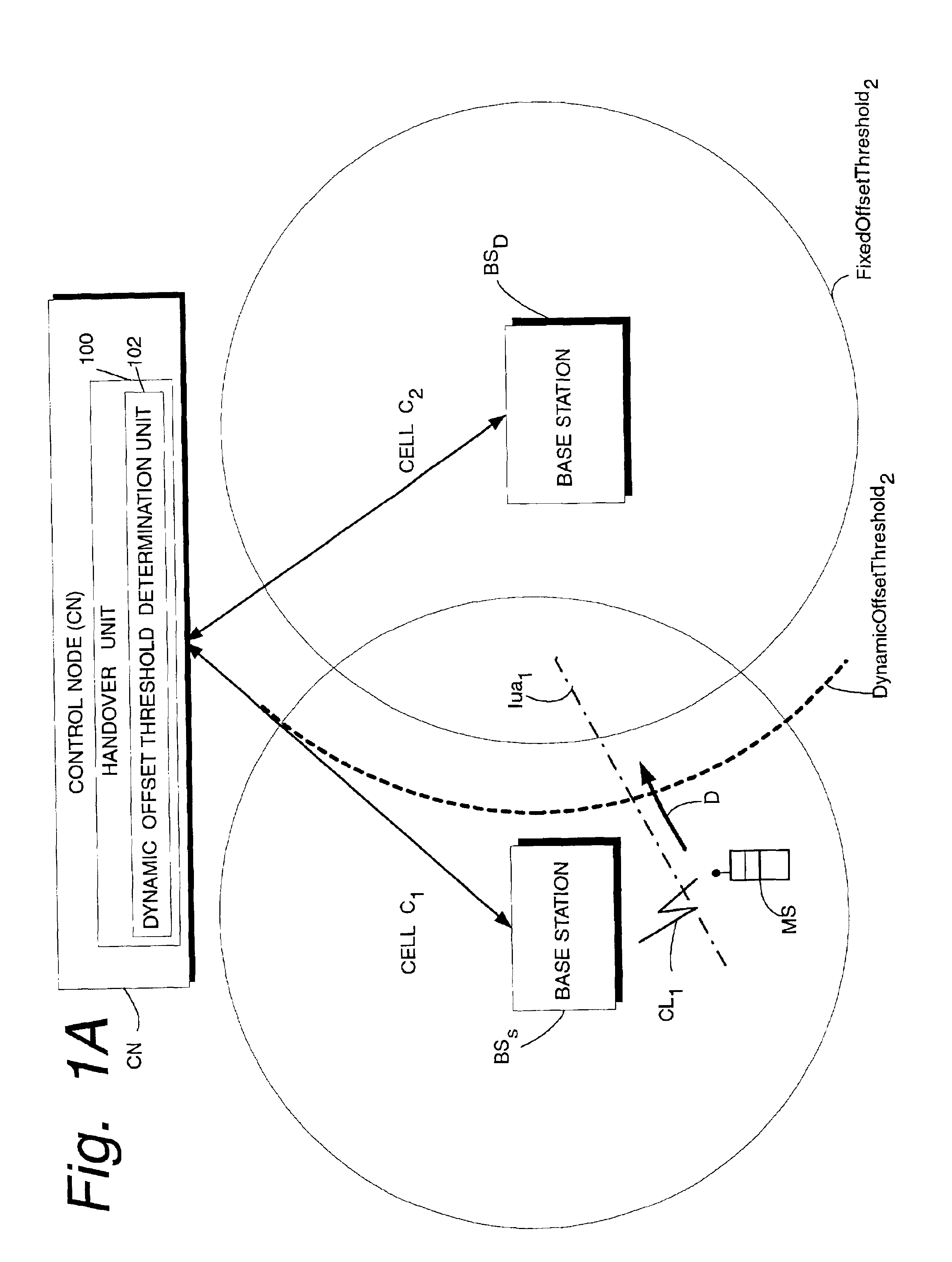
InactiveUS6907245B2Increase capacityReduce riskRadio/inductive link selection arrangementsWireless commuication servicesTime criticalMobile station
A telecommunications system has a source base station (BSS) and a destination base station (BSD), and a handover unit (100) having a dynamic offset threshold determination unit (102) which establishes a dynamic offset threshold for starting soft handover. When the dynamic offset threshold for soft handover is exceeded, a preliminary portion of a handover sequence is initiated at the destination base station. The preliminary portion of the handover sequence is initiated so that a time-critical handover sequence activity (such as L1 uplink synchronization) is well underway, if not completed, by the time the soft handover is actually needed. The dynamic offset threshold for starting handover is based on a probability that the mobile station will engage in the handover. The probability is a statistical probability that handover will actually occur based on handover history of other mobile stations previously and similarly traveling and of the same signal strength. Another portion of the soft handover sequence (e.g., a remaining portion of the soft handover sequence) is initiated when the signal strength from the destination base station as received at the specified mobile station has a predetermined relationship to a fixed offset threshold.
Owner:UNWIRED PLANET
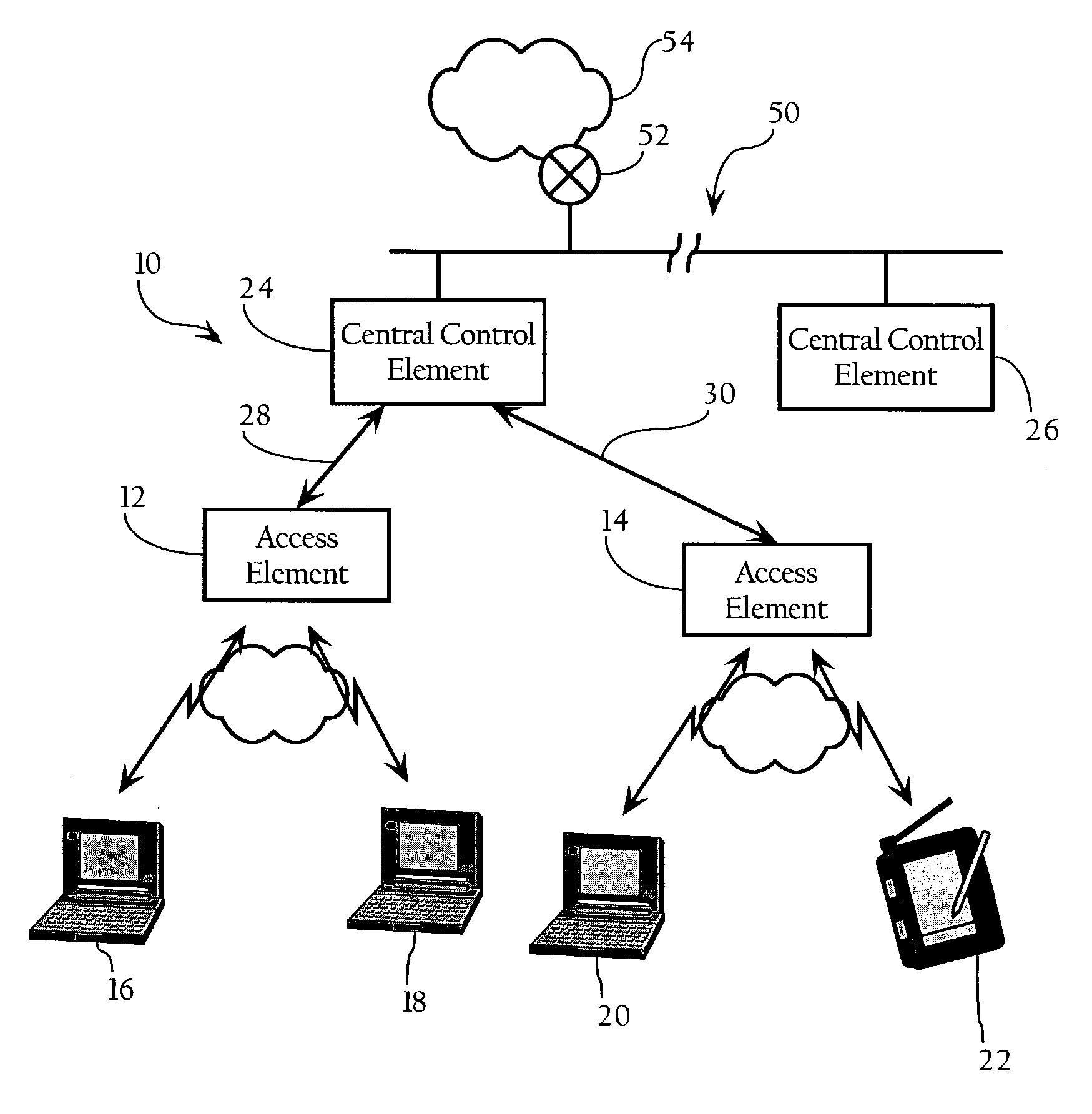
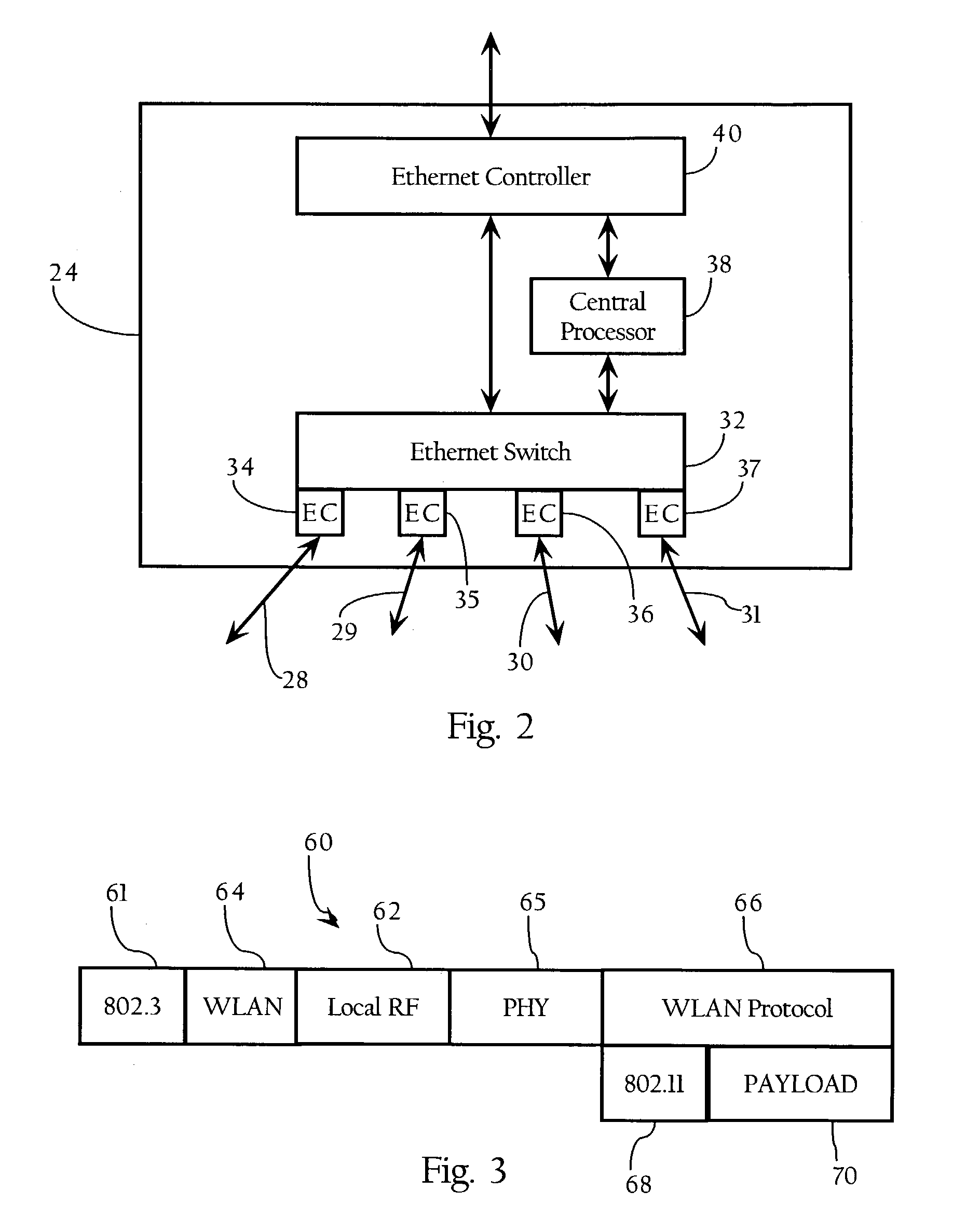
Method and system for hierarchical processing of protocol information in a wireless LAN
ActiveUS7212837B1Improve performanceNetwork traffic/resource managementNetwork topologiesProtocol processingTime critical
In a wireless Local Area Network (WLAN) system, a hierarchical architecture is provided which employs a protocol which divides protocol processing functions between a plurality of substantially identical access elements in which reside time-critical protocol functions, such as acknowledgment and retransmission of packets, and a centralized control element which provides control and management functions related to dynamic configuration of wireless networks, such as processing of network management messages (e.g., authentication and association), load control, channel control, and handoff, processing of physical layer information, and processing of channel characteristics, propagation, interference or noise, for the plurality of access elements in the WLAN without loss of information about the wireless characteristics of the access elements. This hierarchical protocol processing architecture allows the data flow to be centralized for better performance and provides useful access to all the protocol information from the WLAN.
Owner:CISCO TECH INC
Communication device and method of prioritizing transference of time-critical data
InactiveUS20060187836A1Raise transfer toSolve insufficient bandwidthError preventionTransmission systemsData packTime critical
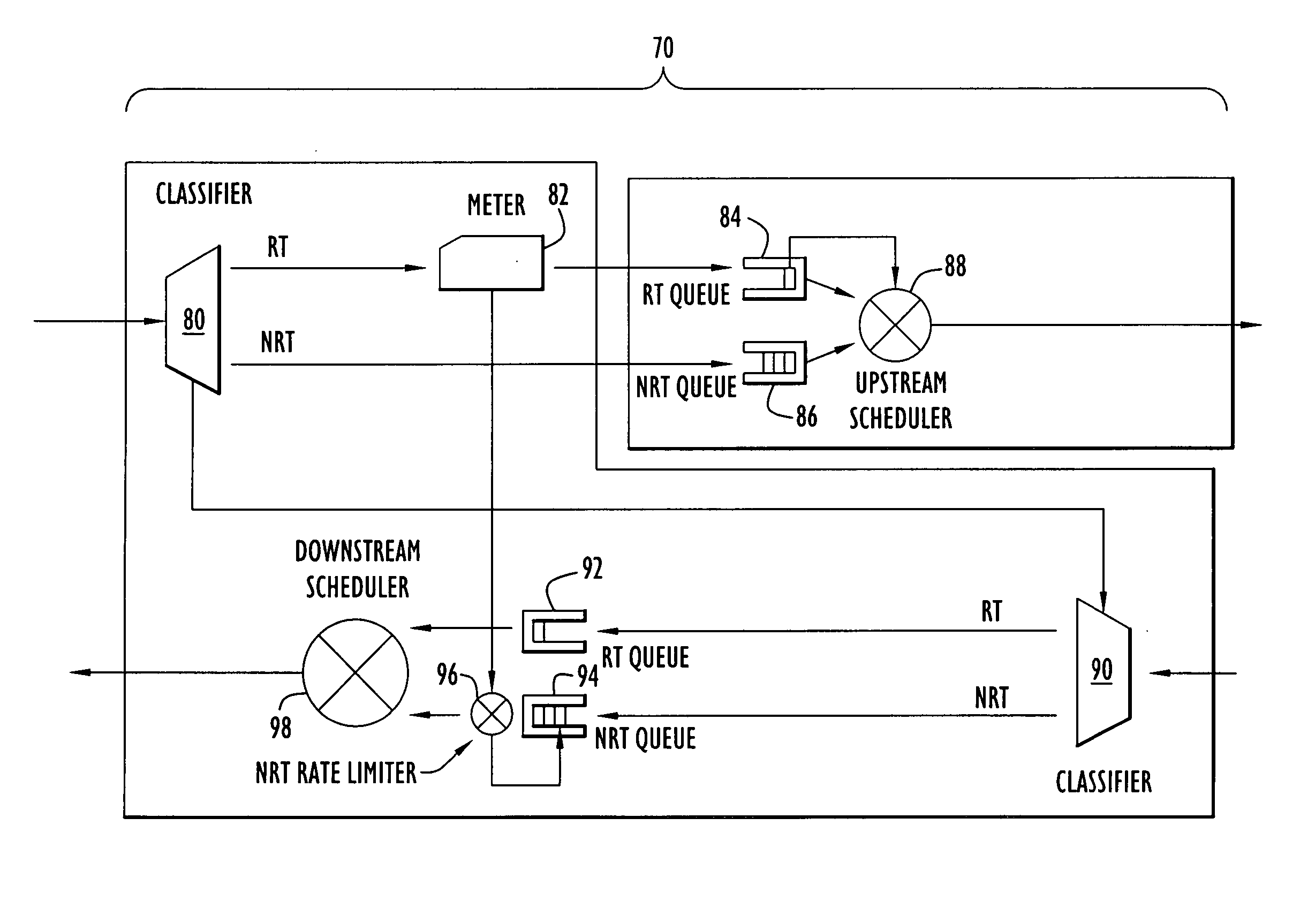
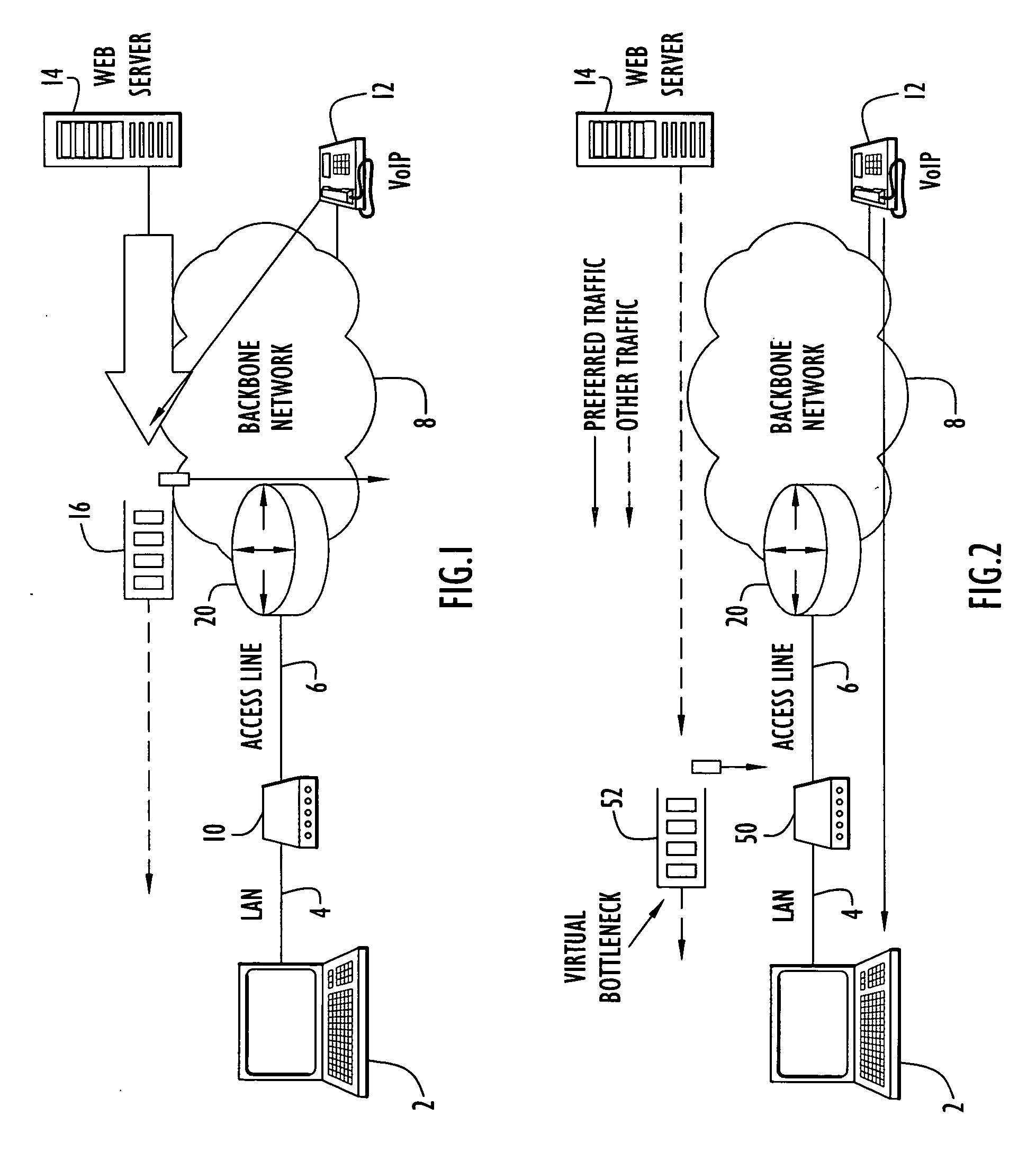
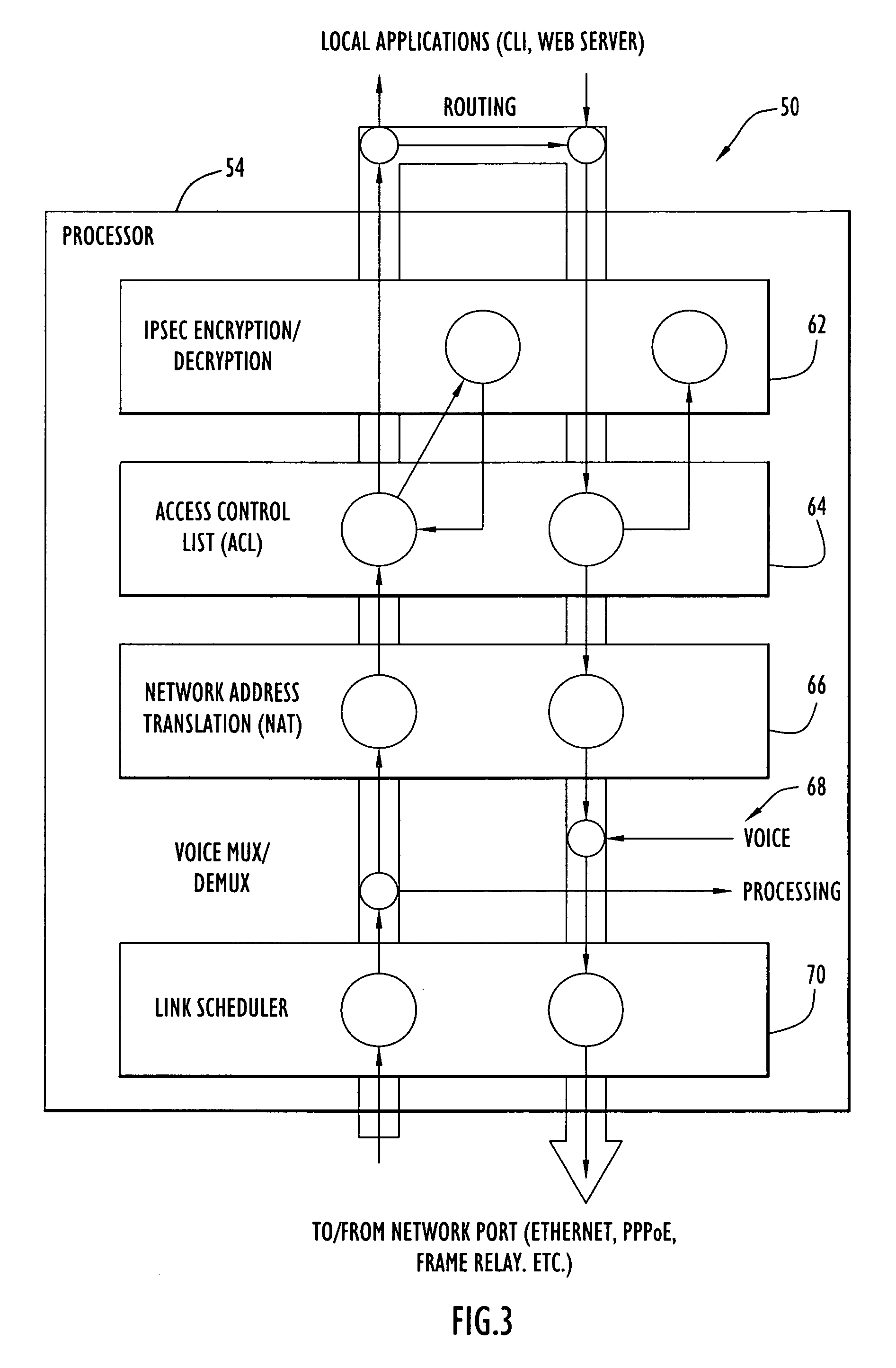
A communication device according to the present invention enhances transfer of time-critical data between one or more LANs and a device (e.g., edge router, etc.) coupled to a backbone network. A virtual bottleneck in the form of a queue is introduced by the communication device at the customer premises or customer end of a backbone network access line where the network congestion or bottleneck resides. The virtual bottleneck delays and / or discards time insensitive traffic prior to time-critical or voice traffic being delayed in the edge router. This is accomplished by the virtual bottleneck queue including a storage capacity or length less than that of a queue utilized by the edge router. A traffic manager or scheduler controlling the virtual bottleneck dynamically adjusts the virtual bottleneck based on the bandwidth required for time-critical packets to ensure sufficient bandwidth is available for those packets.
Owner:PATTON ELECTRONICS
Service deployment infrastructure request provisioning
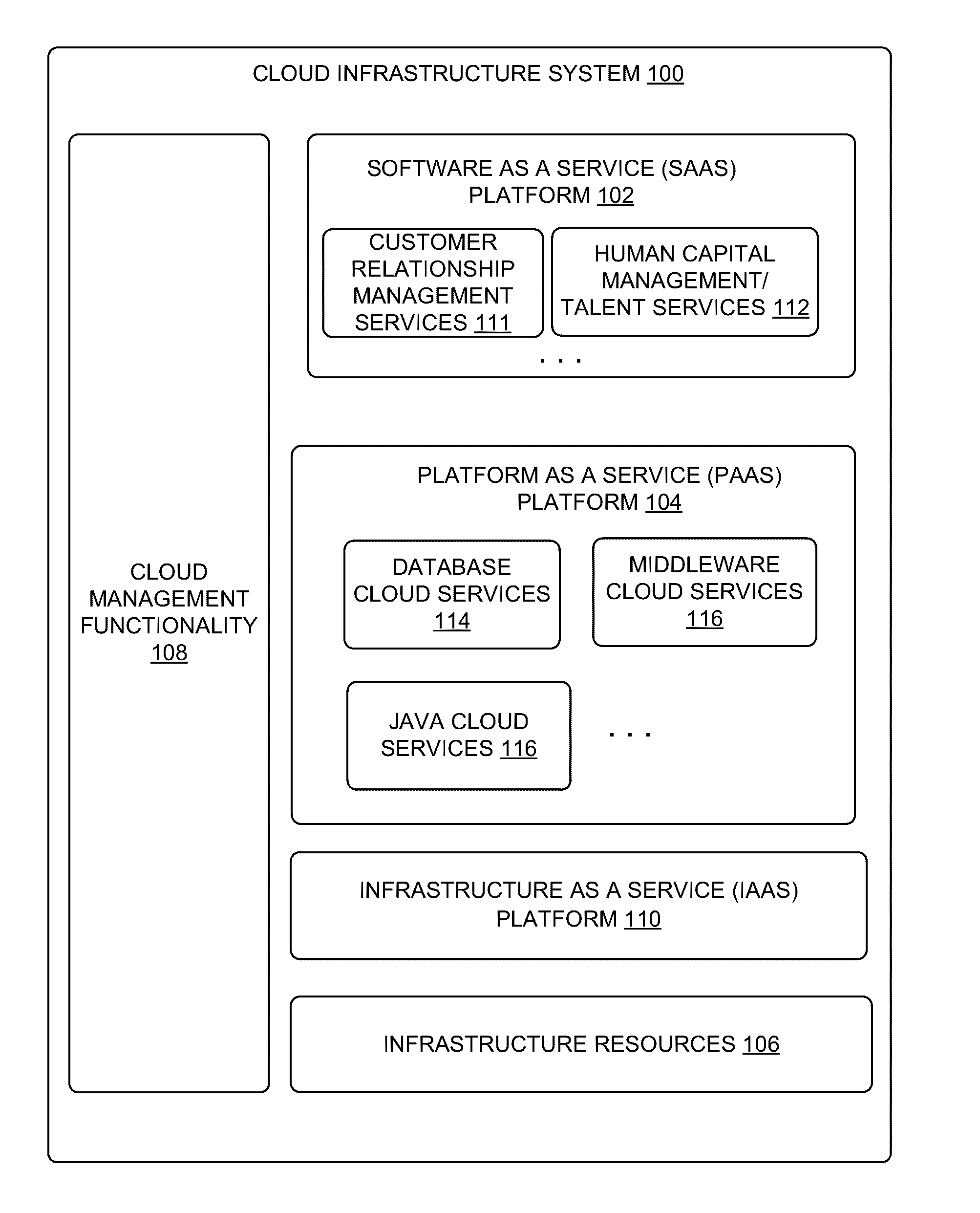
In certain embodiments, a Service Deployment Infrastructure (SDI) request engine is disclosed. The SDI request engine performs the tracking, management and provisioning of services subscribed to by customers of the cloud infrastructure system. The SDI request engine is deployed to process large volumes of provisioning requests and deliver time critical applications for customers. The SDI request engine translates each request into a list of tasks of various sizes based on the requirement and configuration of the request. In some embodiments, the SDI request engine imposes control and management on both request and task levels in order to execute, rollback, retry or fail a task automatically and accurately.
Owner:ORACLE INT CORP
Automatic extension of clock gating technique to fine-grained power gating
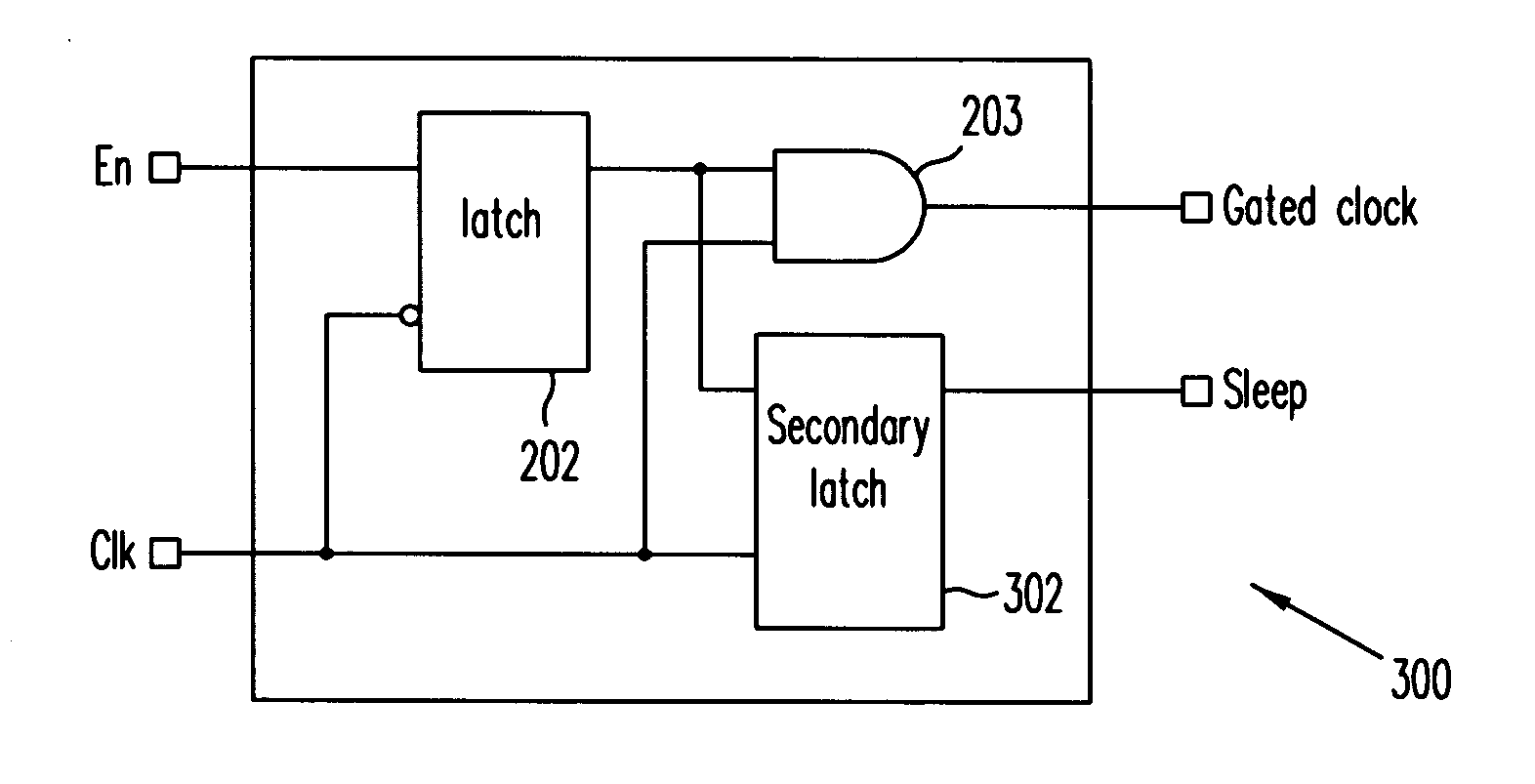
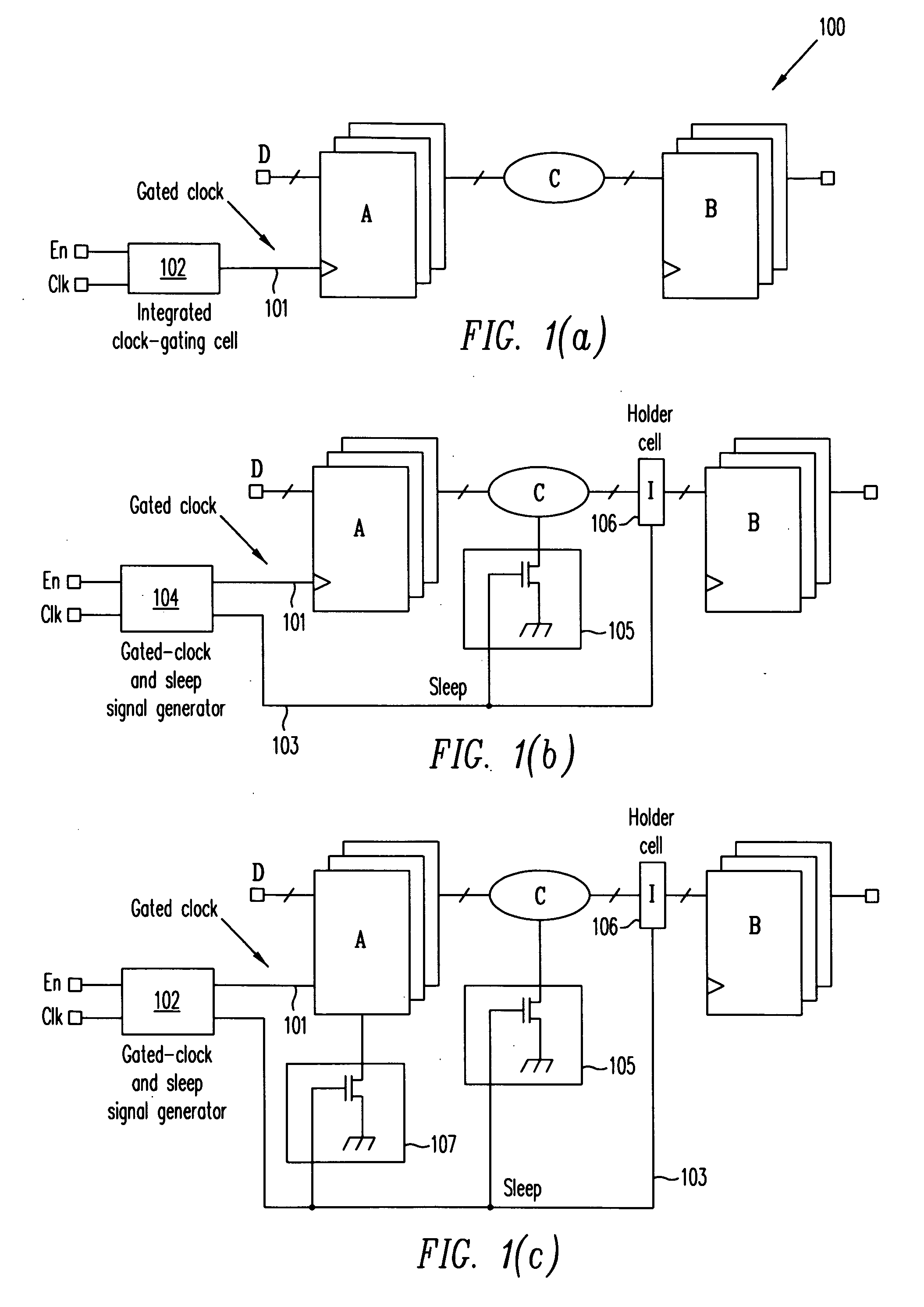
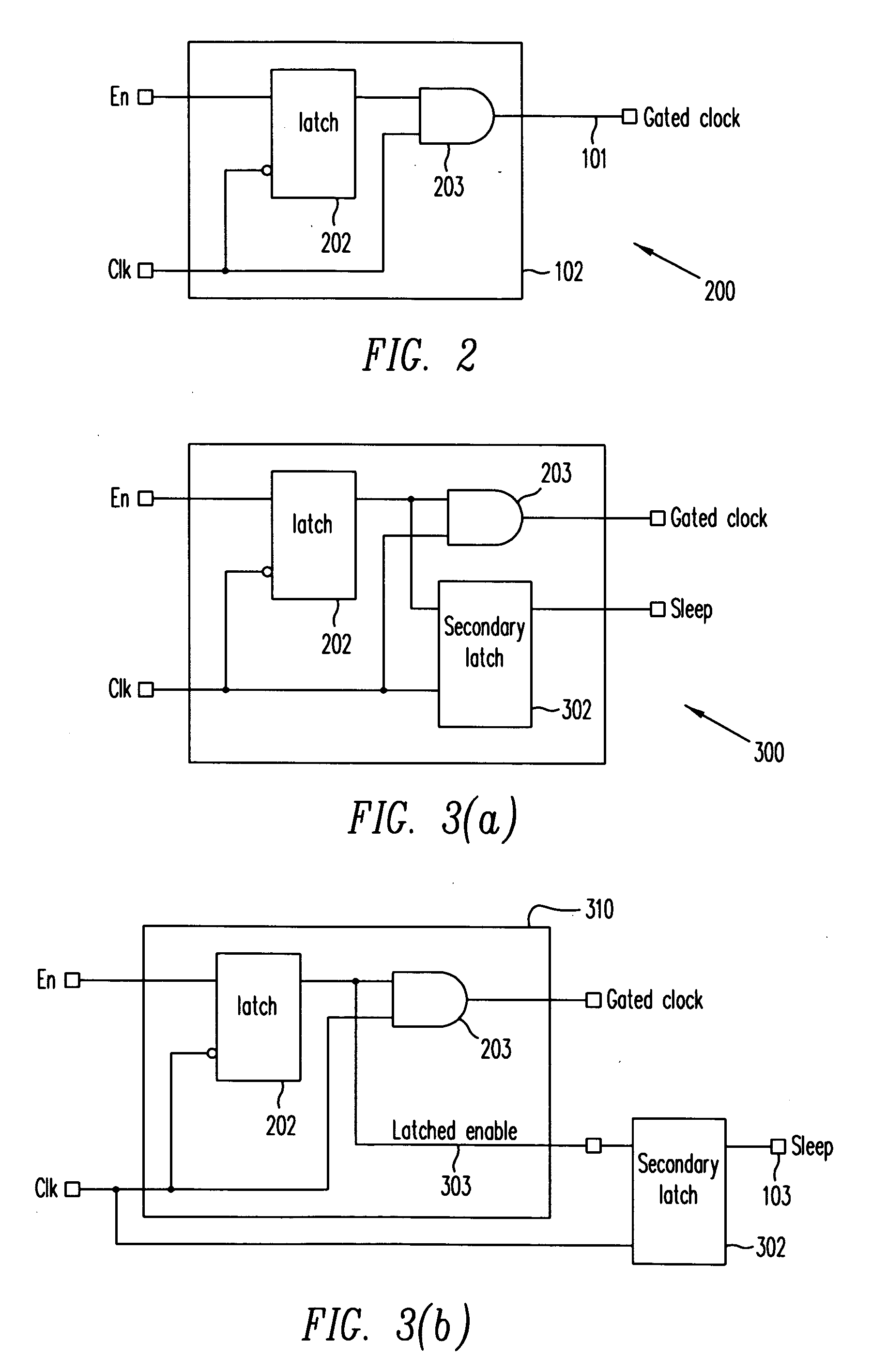
ActiveUS20070024318A1Reduce dynamic power consumptionPower reduction by control/clock signalLogic circuits using elementary logic circuit componentsTime criticalEngineering
A method extends a clock-gating technique to provide a sleep signal for controlling switch circuits that reduce active leakage power. Using this extension of the clock-gating technique, fine-grained power-gating is achieved. The method automatically identifies, at an RTL or a gate level, the logic circuits that can be power-gated. The method of the present invention derives a sleep signal for fine-grained power-gating that may be applicable to both time-critical and non-time-critical designs.
Owner:ANSYS
Method and apparatus implementing a multimedia digital network
InactiveUS20020031144A1Maximal efficiency in transmissionHybrid switching systemsDigital computer detailsData capacityLong term data
A method and apparatus for efficiently managing the allocation of available data capacity on a physically shared digital network among devices connected to that network is disclosed. Also disclosed is a method and apparatus for managing the ongoing timely movement of data on the shared network such that precise long-term data rates are achieved between attached devices with minimal additional buffering. The invention further comprises a method and apparatus which allows the use of any remaining network capacity for non time-critical data movement without the need for centralized access management.
Owner:TIVO SOLUTIONS INC
Wireless lans and neighborhood capture
Overlapped wireless LAN cells in a medium have an equal chance at establishing a session on the medium. A first member station in the first cell transmits a timing packet containing a timestamp value, which is received at a second member station in the second cell. This synchronizes member stations in the first and second cells to interrupt transmissions at a global channel release instant corresponding to the timestamp value. The member stations in the first and second cells then have the opportunity to contend for access to the medium following the global channel release instant, using a slotted CSMA / CA access method. Each of the member stations in the first and second cells has a superframe clock that is synchronized based on the timestamp value, thereby establishing a periodic global channel release instant during each of a plurality of periodic superframes. The member stations can then periodically interrupt transmissions at the periodic global channel release instant to contend for the medium. The periodic global channel release instant occurs at intervals that are sufficiently close to meet delay and jitter restrictions for time-critical voice and video applications.
Owner:AT & T INTPROP II LP
System and method for automated tuning of program execution tracing
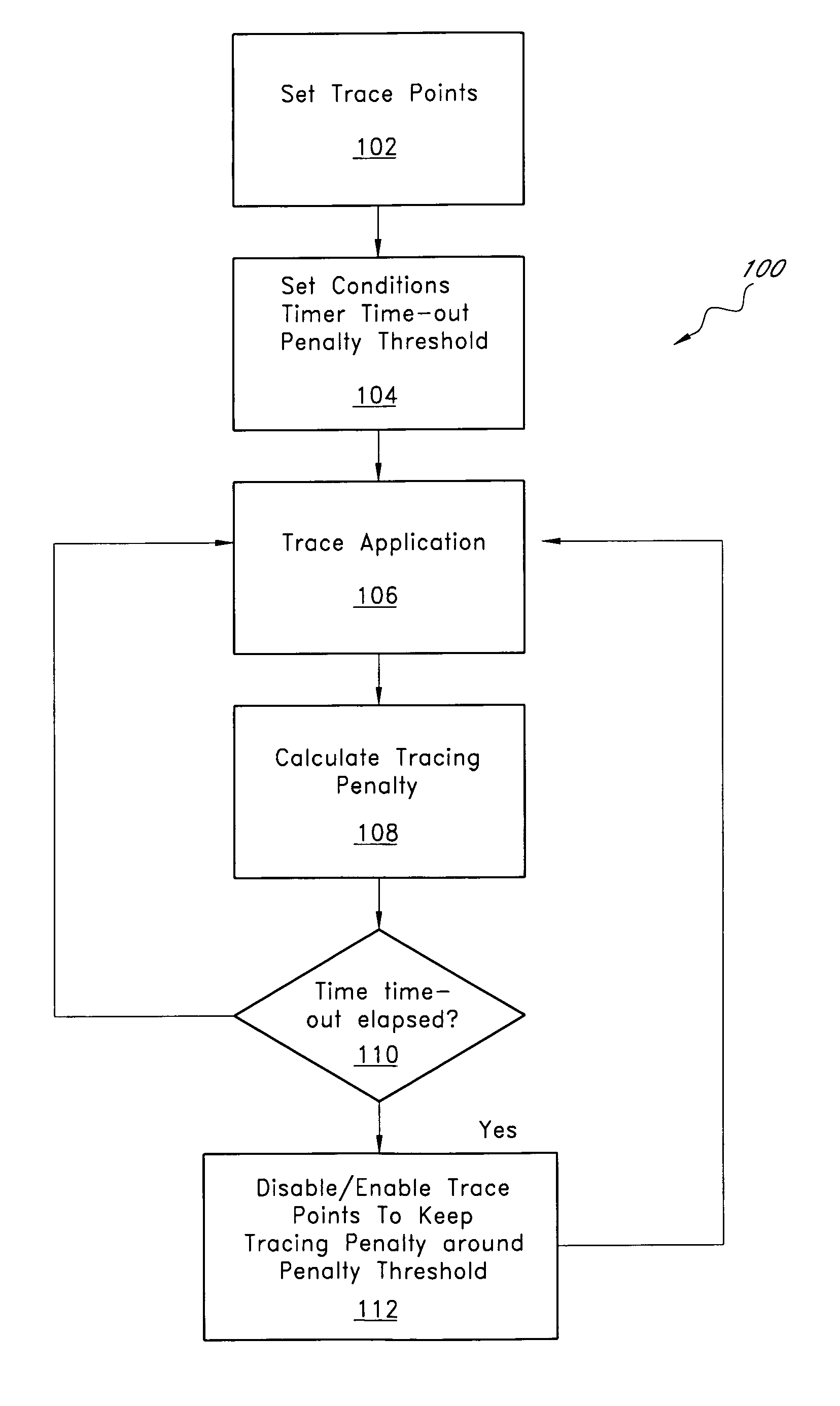
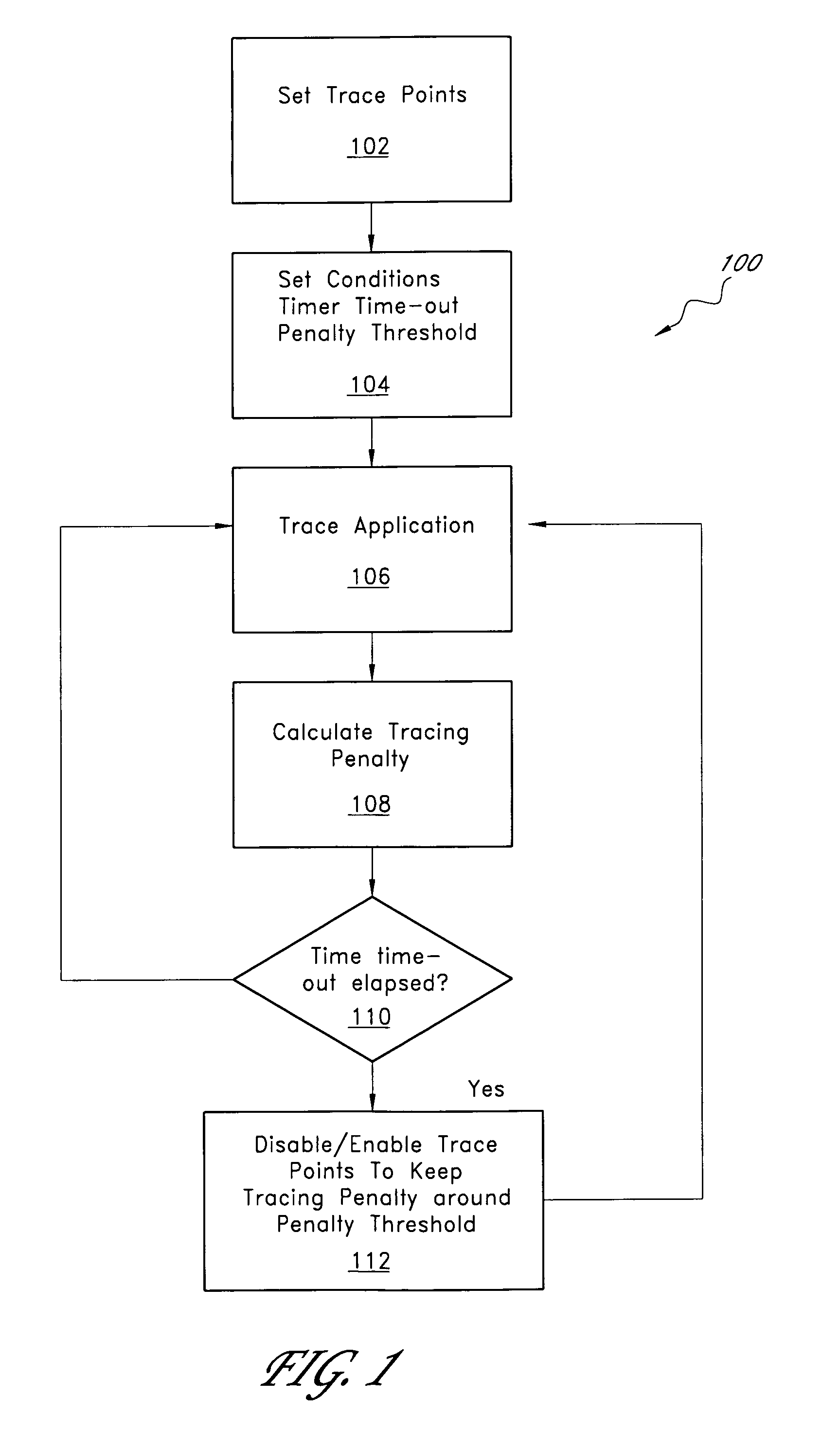
ActiveUS7827539B1Simple processMaintain balanceError detection/correctionSpecific program execution arrangementsTime criticalParallel computing
A tracing system that provides automated tuning of execution tracing by adjusting the collection of trace data is described. In one embodiment, the user sets an initial tracing profile for a tracing program. In addition, the user sets an upper limit for the tracing performance penalty. The auto-tuning system monitors the performance penalty induced by tracing and, when the performance impact is excessive, removes trace points that are causing the most impact on performance. Auto tuning is especially useful for performing software recording in mission-critical and / or time-critical applications, such as servers, real-time applications, etc. The system typically adjusts relatively quickly such that most users do not feel the influence of the tracer.
Owner:BMC SOFTWARE ISRAEL LTD
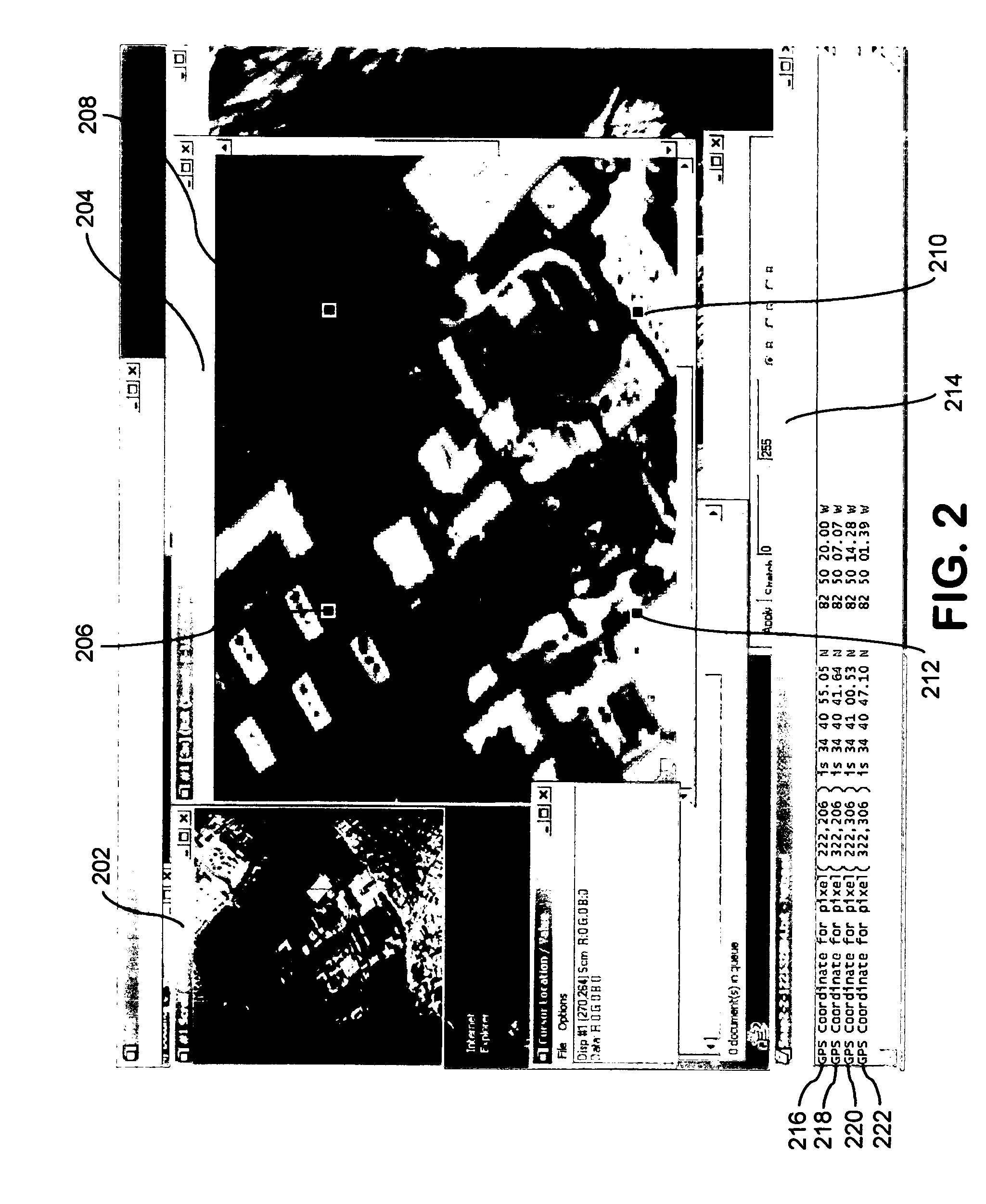
Airborne imaging spectrometry system and method
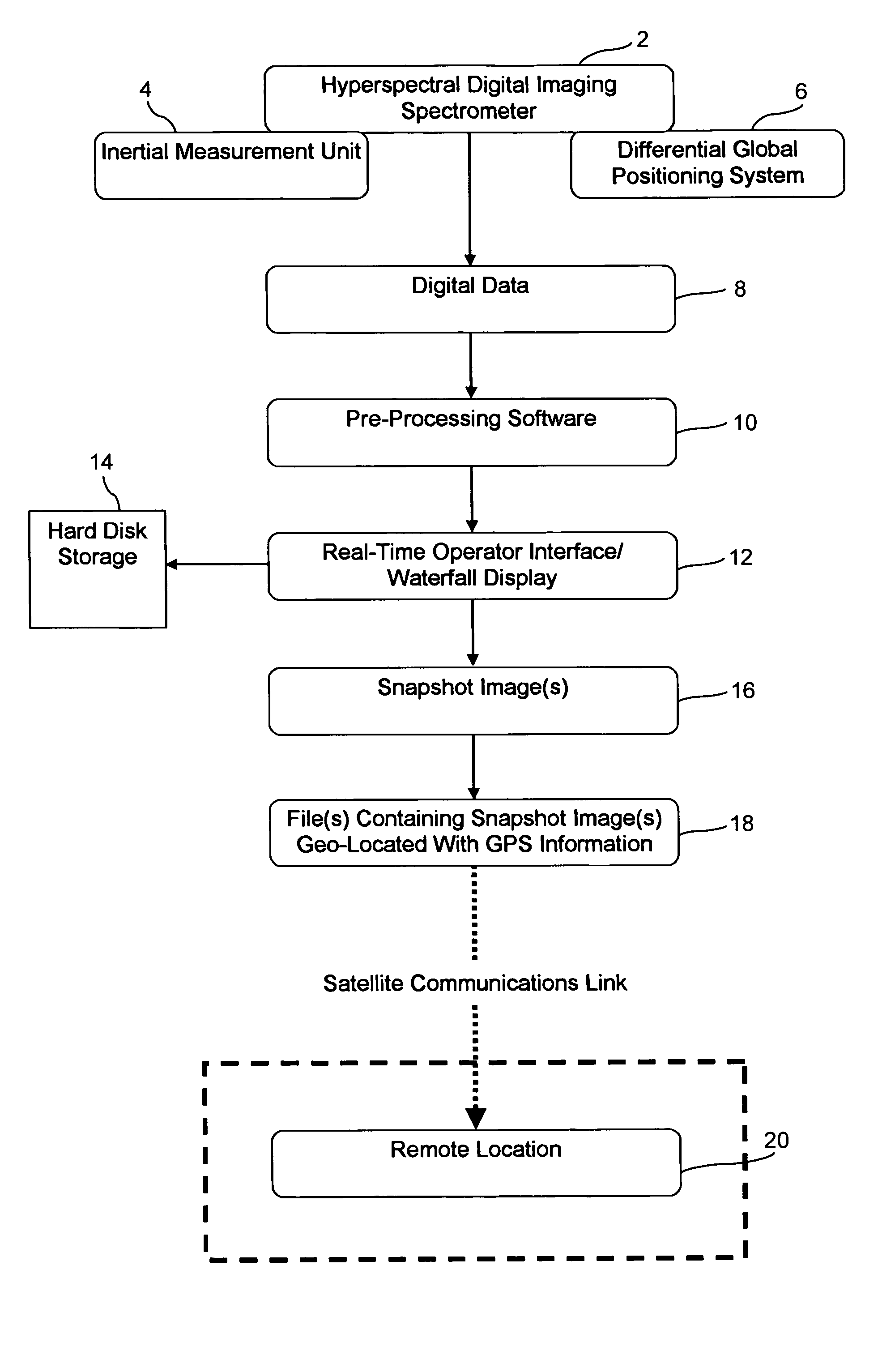
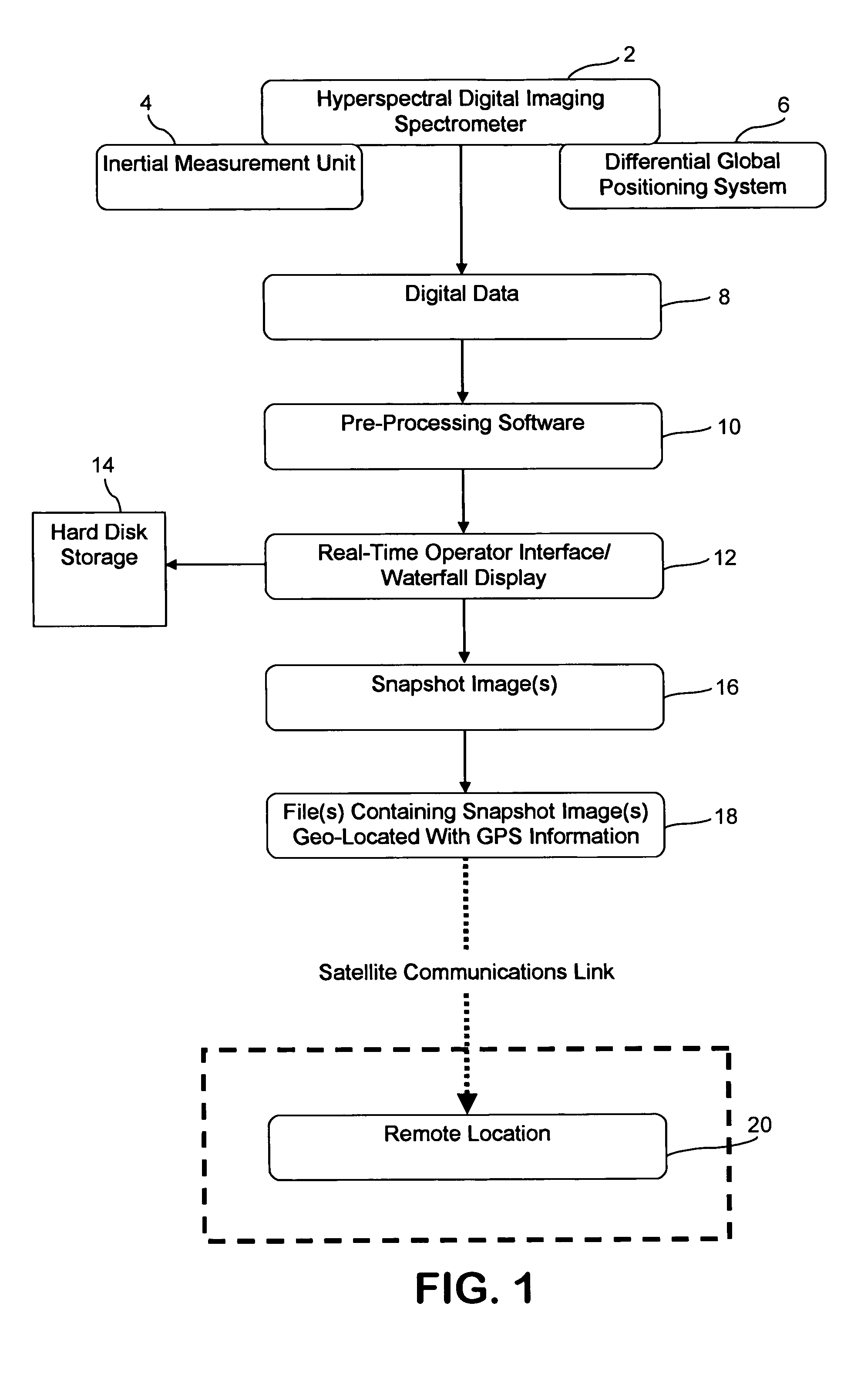
InactiveUS20050104771A1Spectrum investigationMaterial analysis by optical meansVegetationTime critical
The present invention generally relates to an airborne imaging spectrometry method and system. According to the present invention, a digital airborne imaging spectrometer is provided aboard an aircraft and is used to collect hyperspectral imagery of an area of interest while the aircraft flies over the area of interest. The method and system of the present invention combine (1) real-time display, aboard an aircraft, of the hyperspectral imagery being collected for an area of interest below the aircraft with (2) transmission of such hyperspectral imagery to a remote location, wherein such imagery is received at the remote location in near real-time. When hyperspectral imagery and related data are received from the aircraft at the remote location, the transmitted hyperspectral imagery and related data are useful at the remote location in time-sensitive or time-critical decision making. Forest fires, infestations of vegetation, and law enforcement scenarios such as counter-narcotic operations are examples of situations in which time-sensitive or time-critical decision making may be necessary and in which the airborne imaging spectrometry system and method of the present invention may be used.
Owner:SPECTROTECH
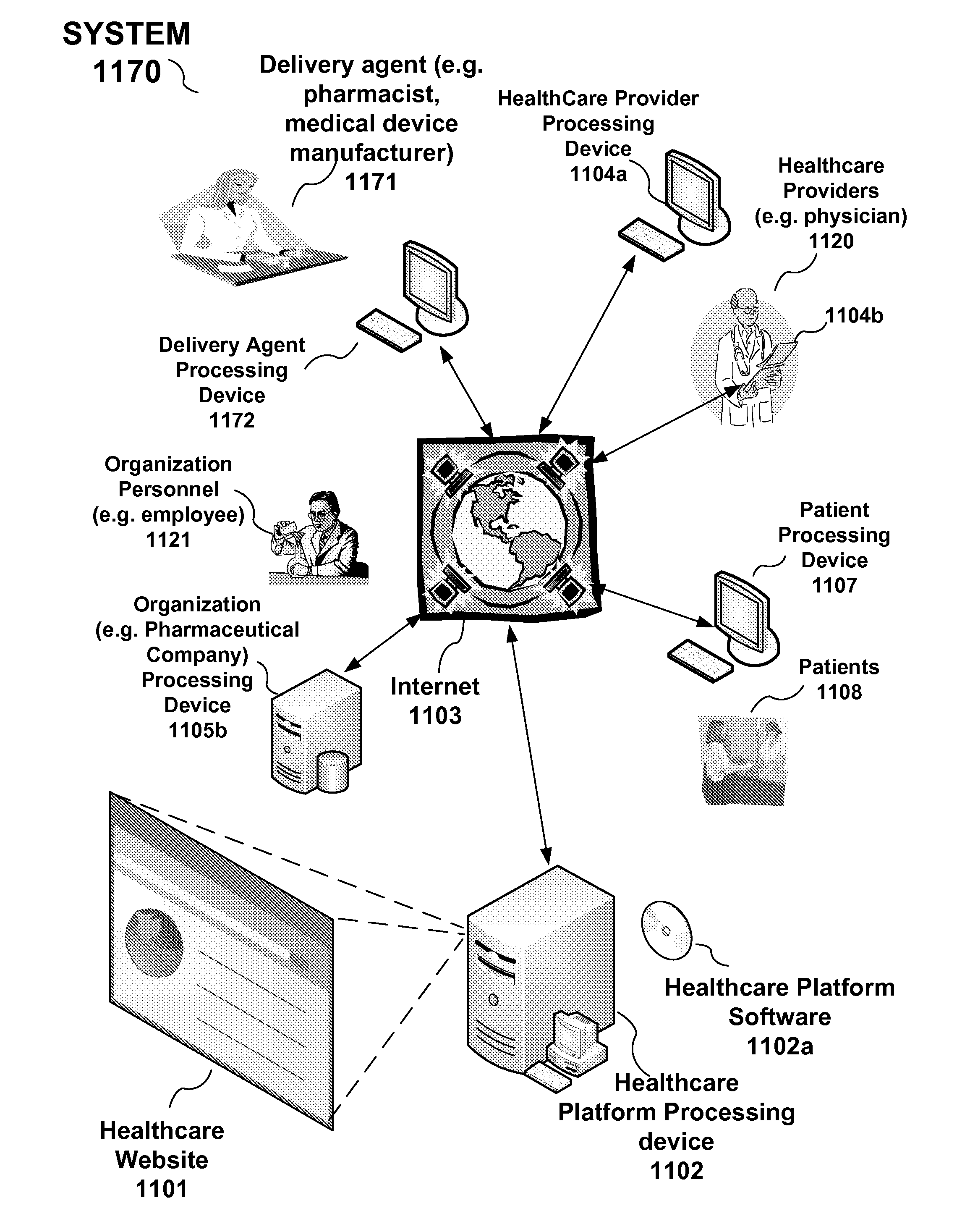
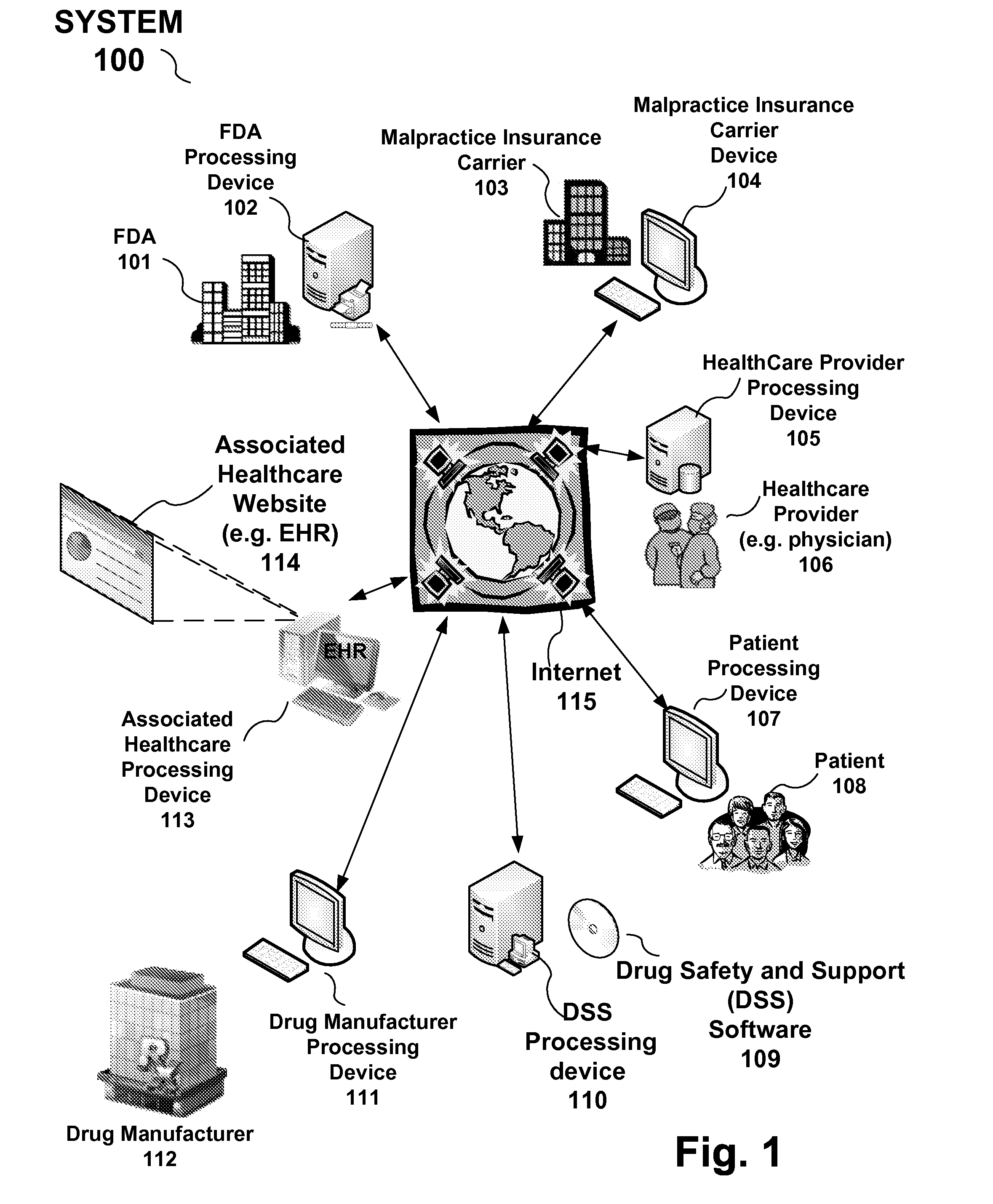
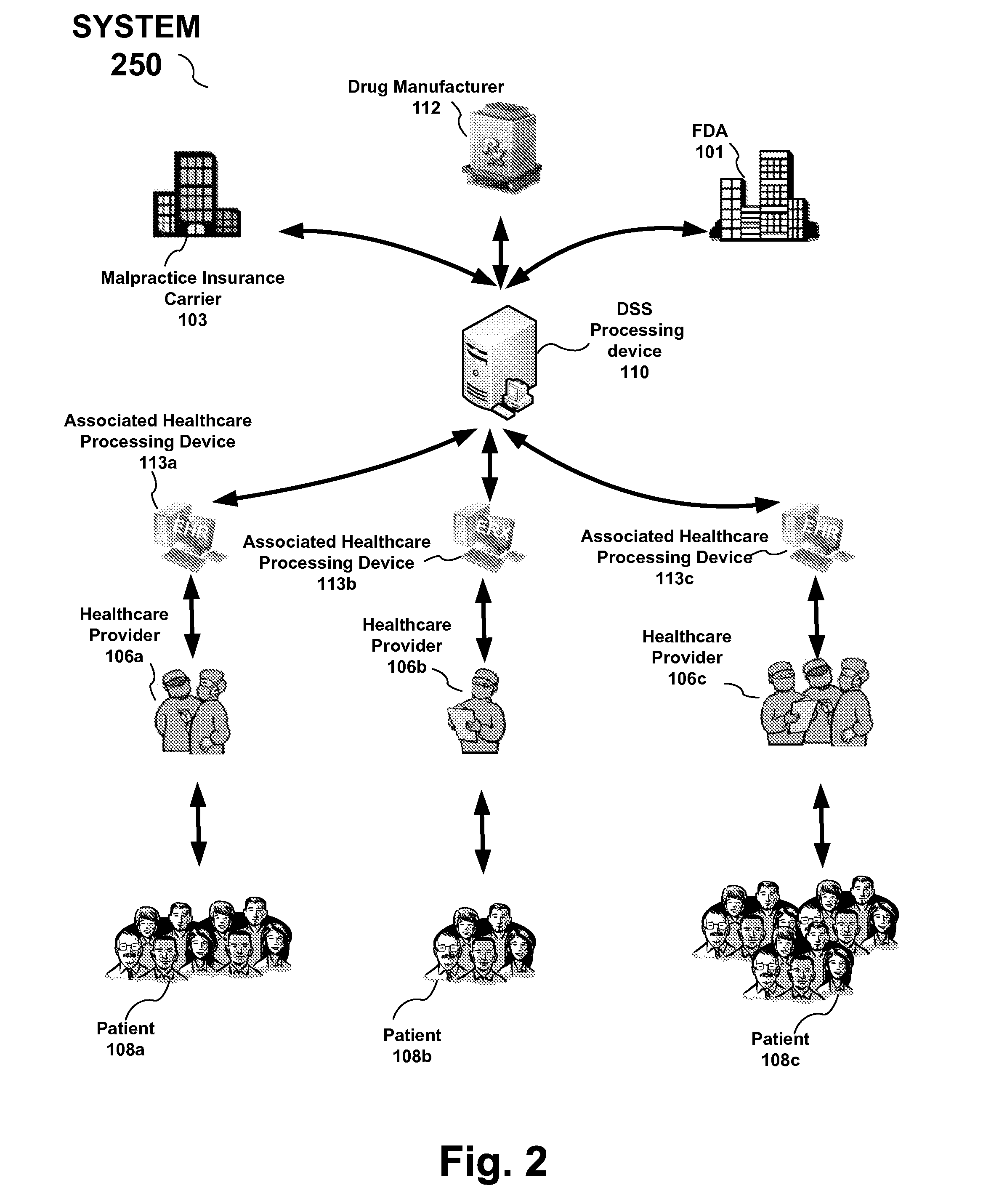
Drug and medical device safety and support information reporting system, processing device and method
A reporting system, including a processing device, and method provides drug and medical device safety and support information during a workflow of a healthcare provider. The system and method electronically acquires, maps, generates, compiles, verifies and transfers in real time critical information regarding particular drugs and medical devices required by healthcare providers, such as a prescriber, at the optimum time in which the healthcare provider needs the information. In an embodiment, the drug and medical device safety information is accessed from an associated healthcare website, such as an Electronic Health Record (EHR) web site, during a prescription stage in the healthcare providers workflow for a particular patient. The system and method also includes an adverse reporting system that allows for the drug and medical device safety information to be updated and accurately reported in an efficient and up to date manner. In an embodiment, a healthcare provider reports an adverse event or reaction to a drug by a patient during a workflow at a EHR web site. The adverse event information is forwarded to the Federal Drug Administration (FDA) and the particular drug manufacturer which may update the corresponding drug information. The updated drug information may then be reported by the system to insure that healthcare providers receive the most current, accurate and complete safety information during a workflow. In embodiments, the updated drug and medical information may be provided to malpractice insurance carriers to further ensure safety.
Owner:PDR NETWORK
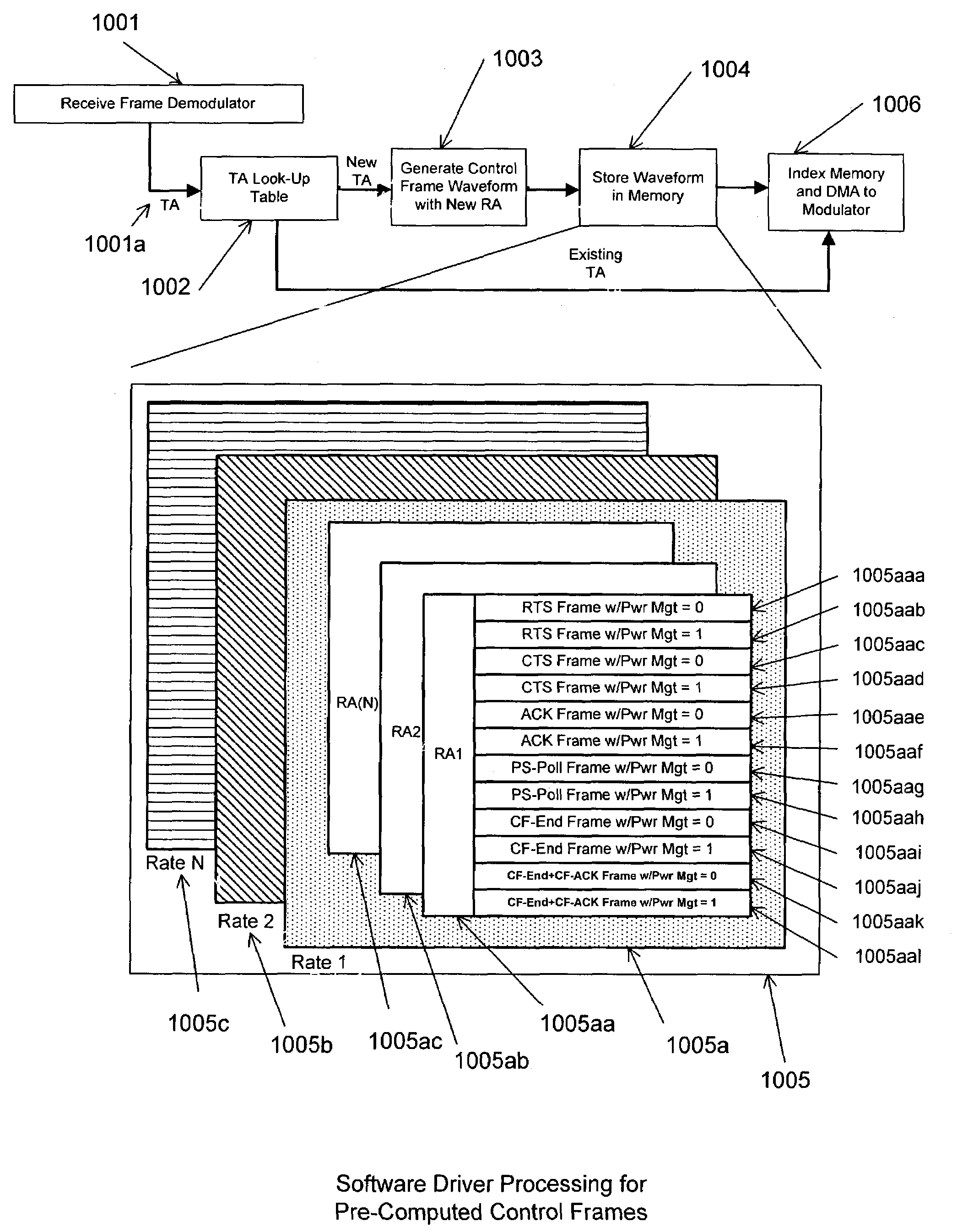
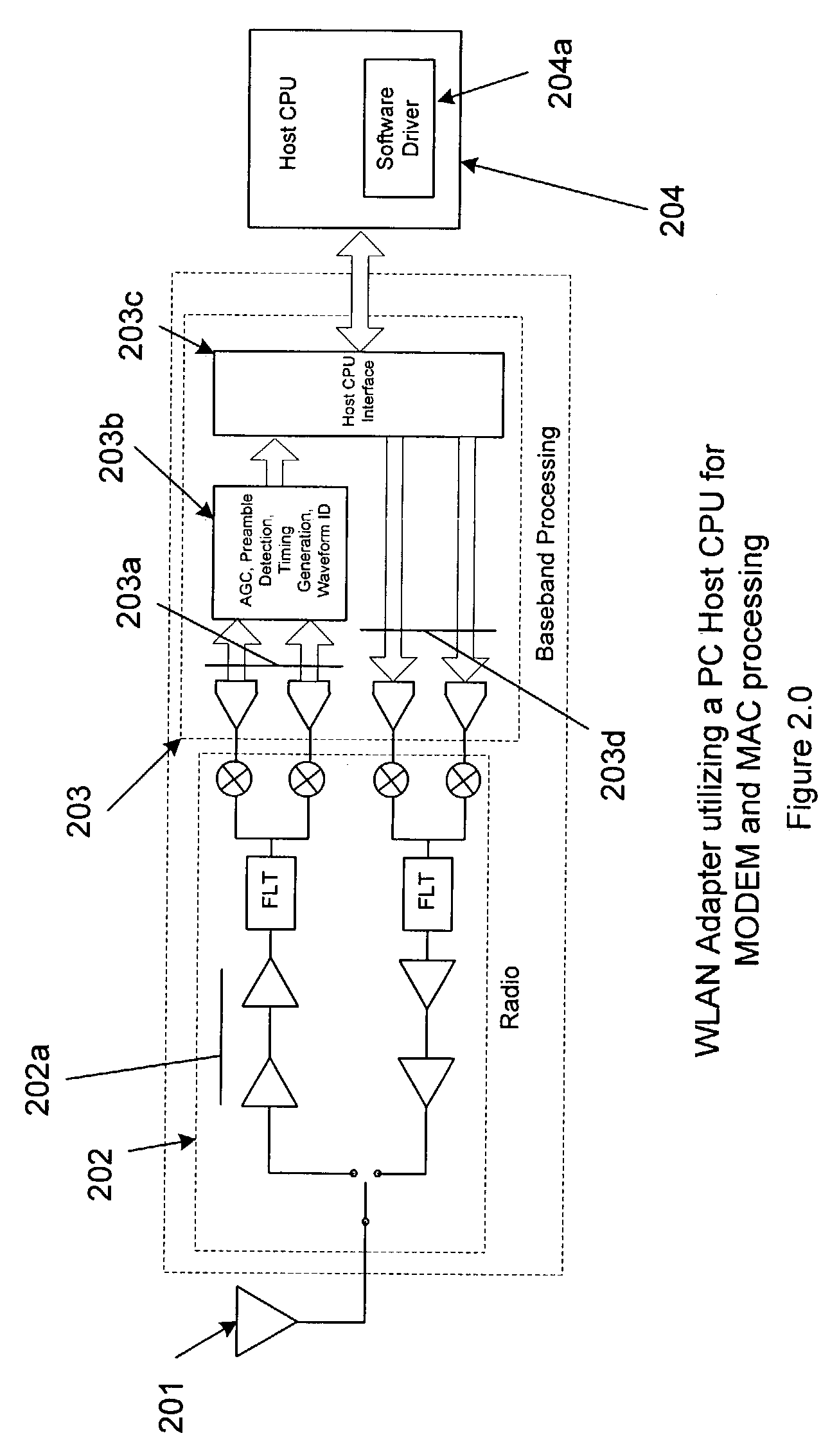
Method for minimizing time critical transmit processing for a personal computer implementation of a wireless local area network adapter
ActiveUS7269153B1Low costLoad minimizationPower managementTime-division multiplexDigital analog converterNetwork packet
A personal computer's (PC) microprocessor is used to provide both the physical layer (PHY) and media access control (MAC) processing functions required to implement a wireless local area network (WLAN) adapter. This technique uses the microprocessor within the personal computer to pre-compute the time critical PHY waveforms required to respond to received packets. For instance, the acknowledge (ACK) waveform required to respond to a received WLAN packet is pre-computed and stored in the PC memory. Upon receipt of a valid packet, the samples of the ACK waveform are transferred from the PC memory to a digital to analog converter (DAC). The DAC generates the transmit waveform required for the radio modulator. By pre-computing the transmit waveform samples, the required loading on the PC microprocessor is reduced during time critical periods.
Owner:CONEXANT SYST INC +1
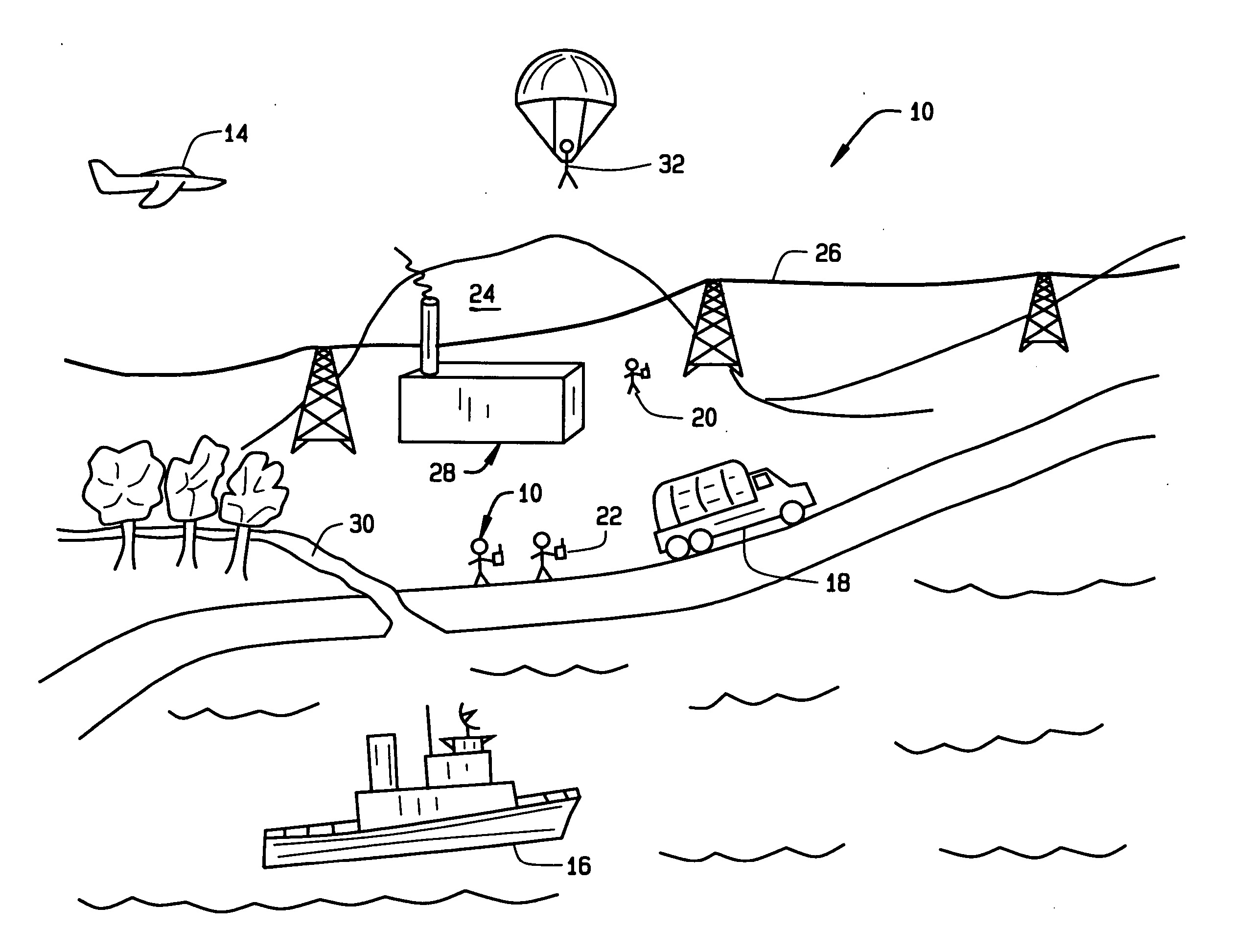
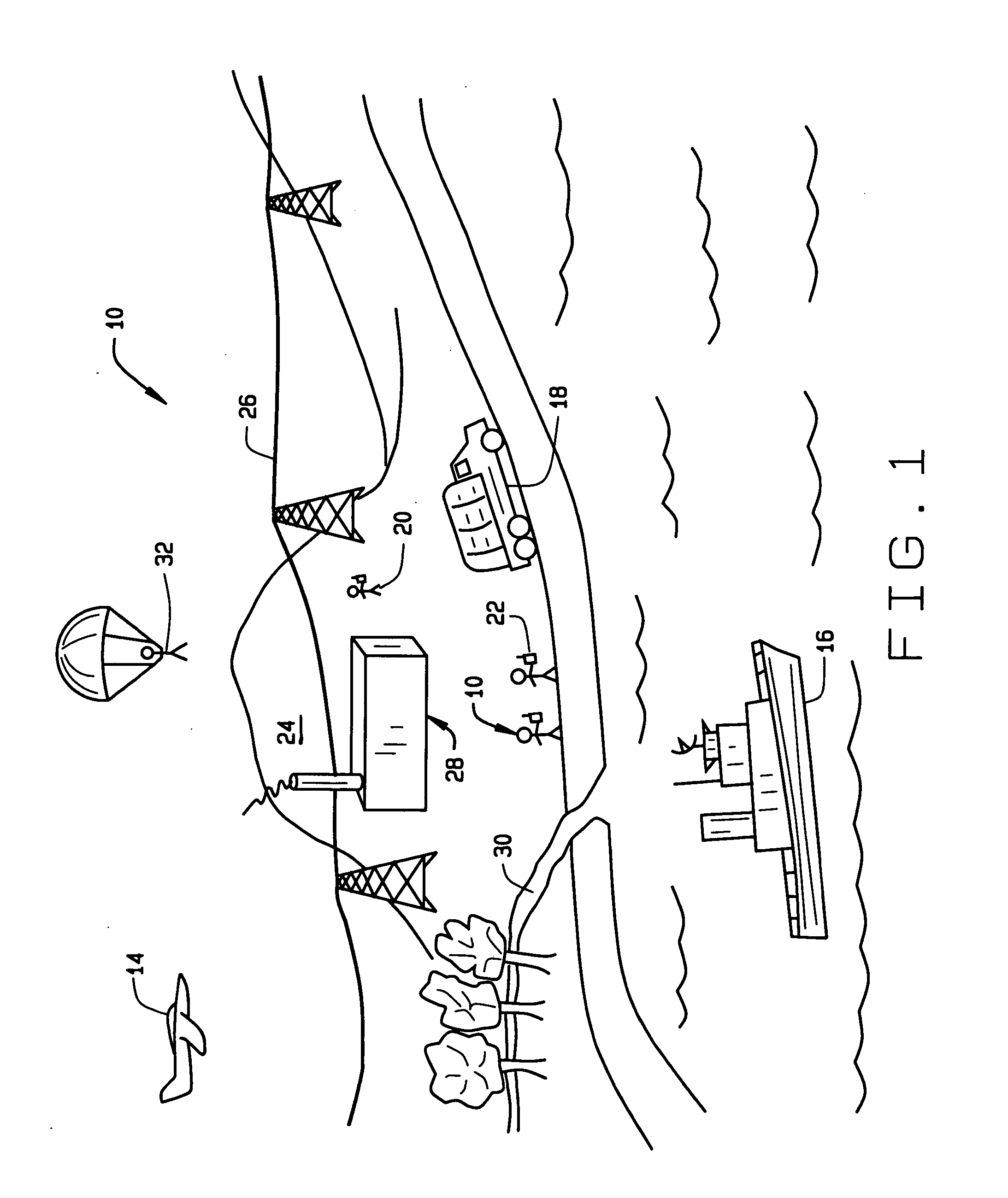
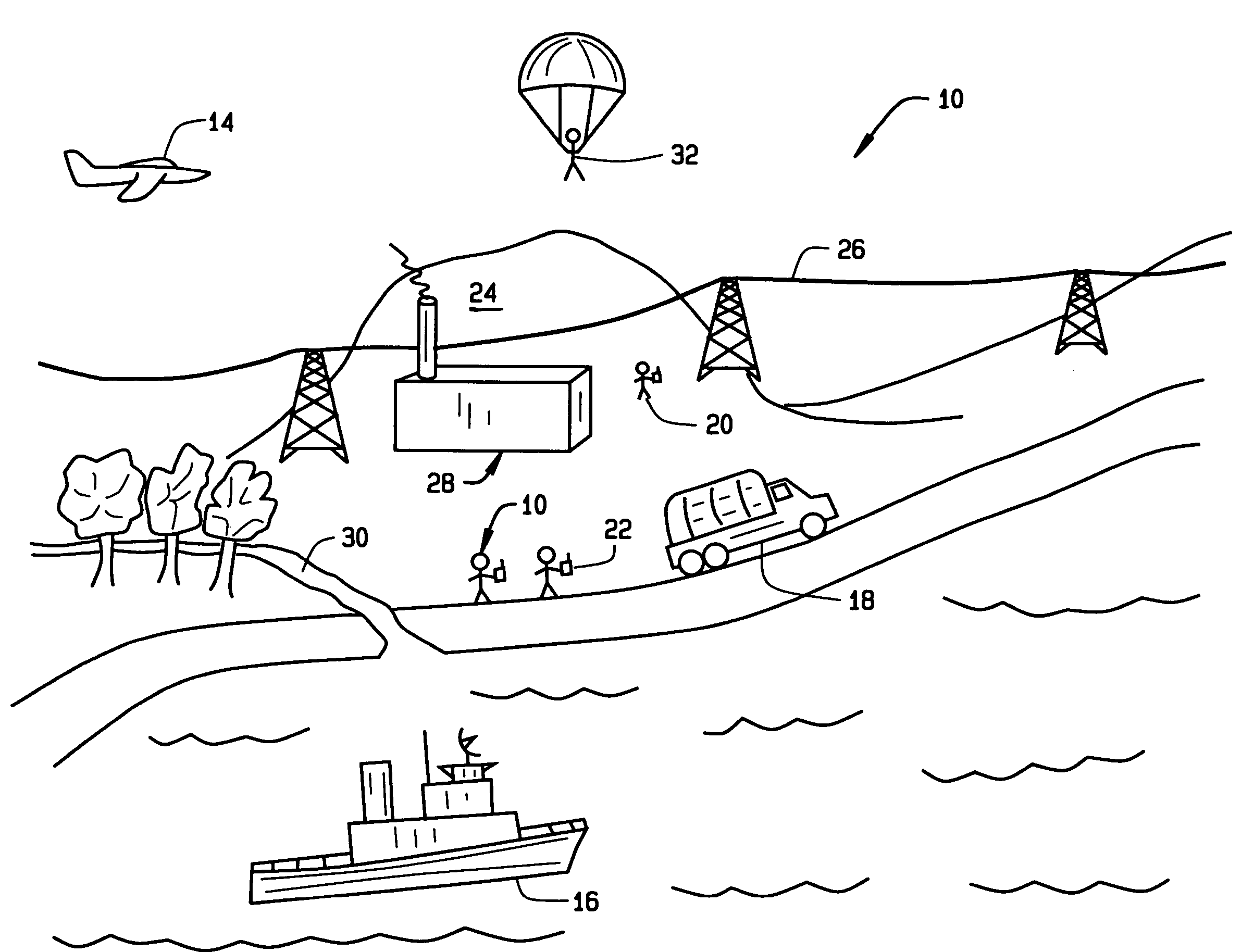
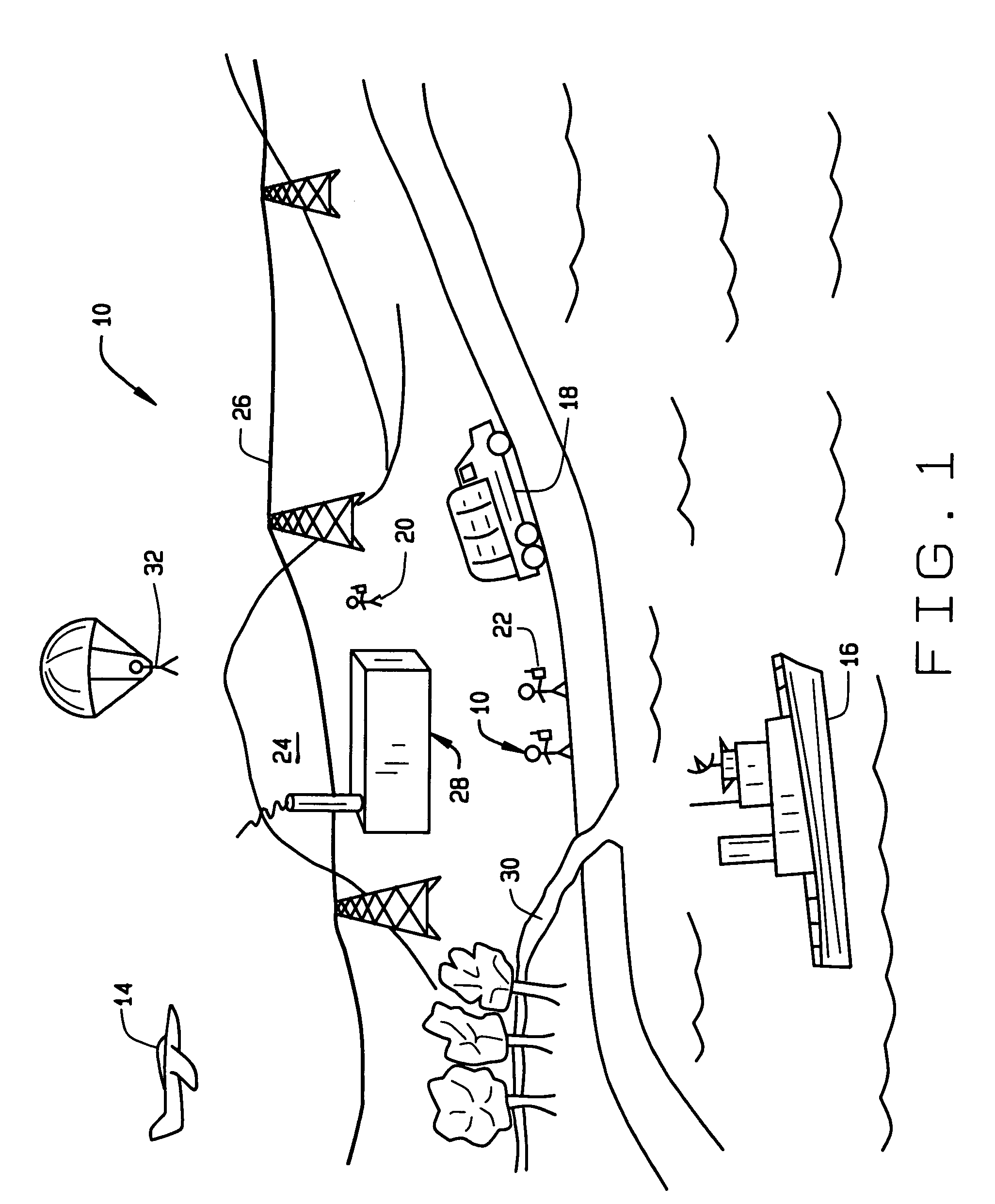
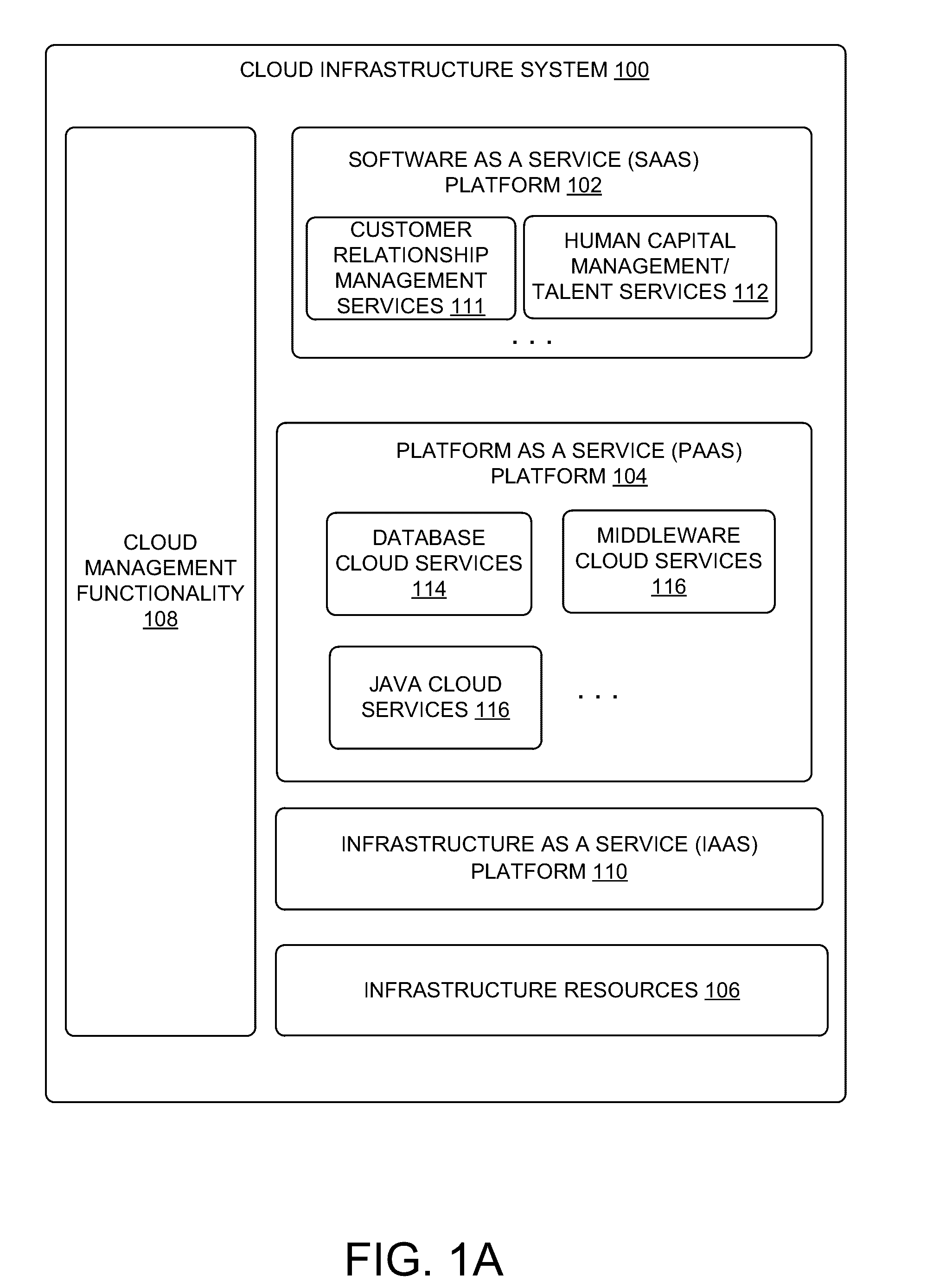
Wireless LAN architecture for integrated time-critical and non-time-critical services within medical facilities
InactiveUS20040109429A1Avoiding lockstep interferenceEasy to receiveSurgeryData switching by path configurationAntenna designDevice type
A wireless local area network (WLAN) system comprises multiple access points that are distributed throughout a medical facility to provide wireless access to a hardwired network. The access points implement multiple WLAN protocols, including a realtime protocol for realtime patient monitoring (telemetry) and a standard WLAN protocol (such as IEEE 802.11 within an ISM band) for providing general-purpose wireless access. Some or all of the access points preferably implement both WLAN protocols such that the different WLANs and wireless device types share network access resources. Some or all of the access points may also include RF location-tracking modules which may be used to track locations of patients, hospital personnel, capital equipment, and / or disposable medical supplies. Also disclosed are an antenna design which may be used with the access points to improve-reception (particularly for patient monitoring), and a TDMA timeslot rotation method for avoiding lockstep interference between access points that operate on the same channel.
Owner:GE MEDICAL SYST INFORMATION TECH
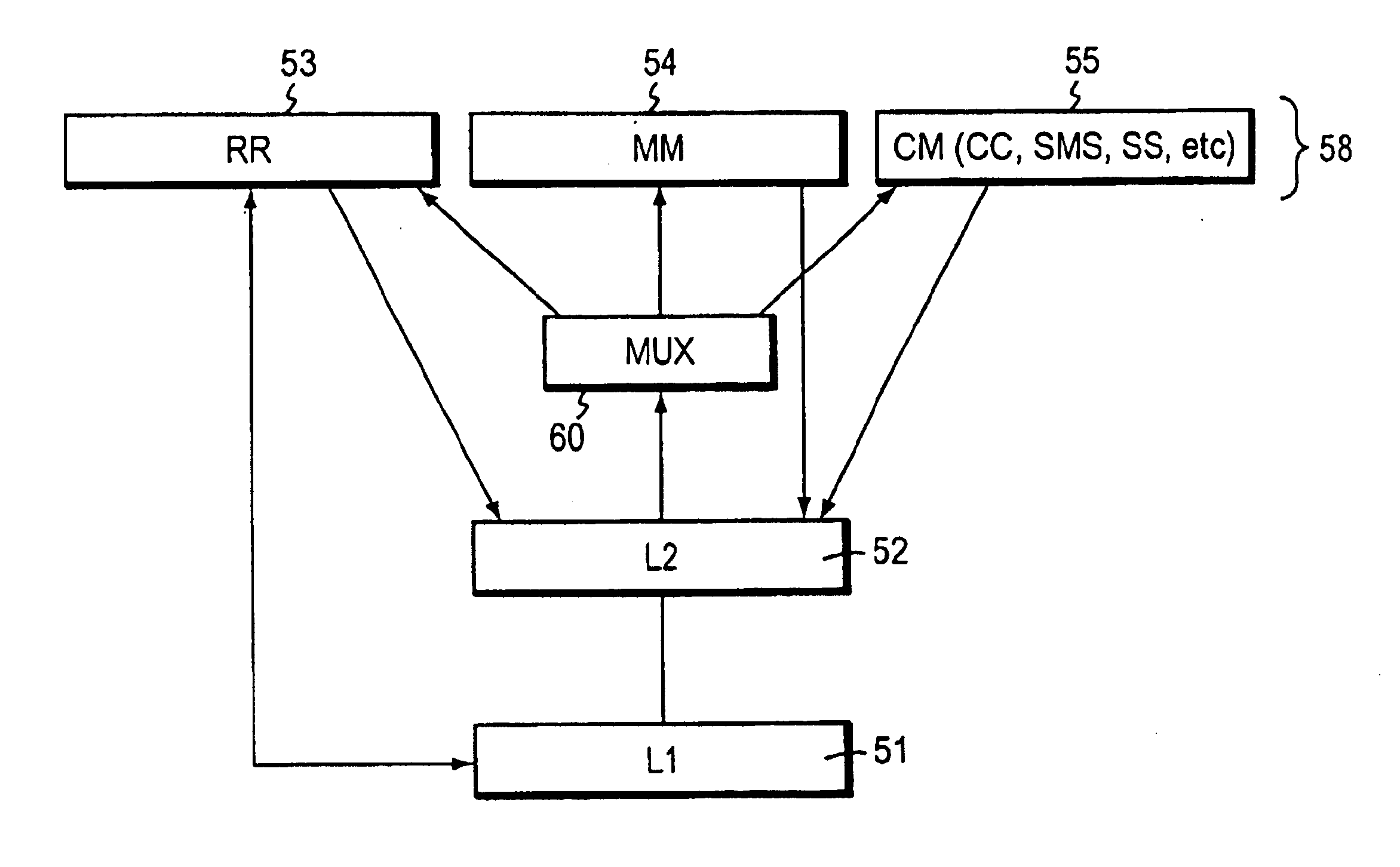
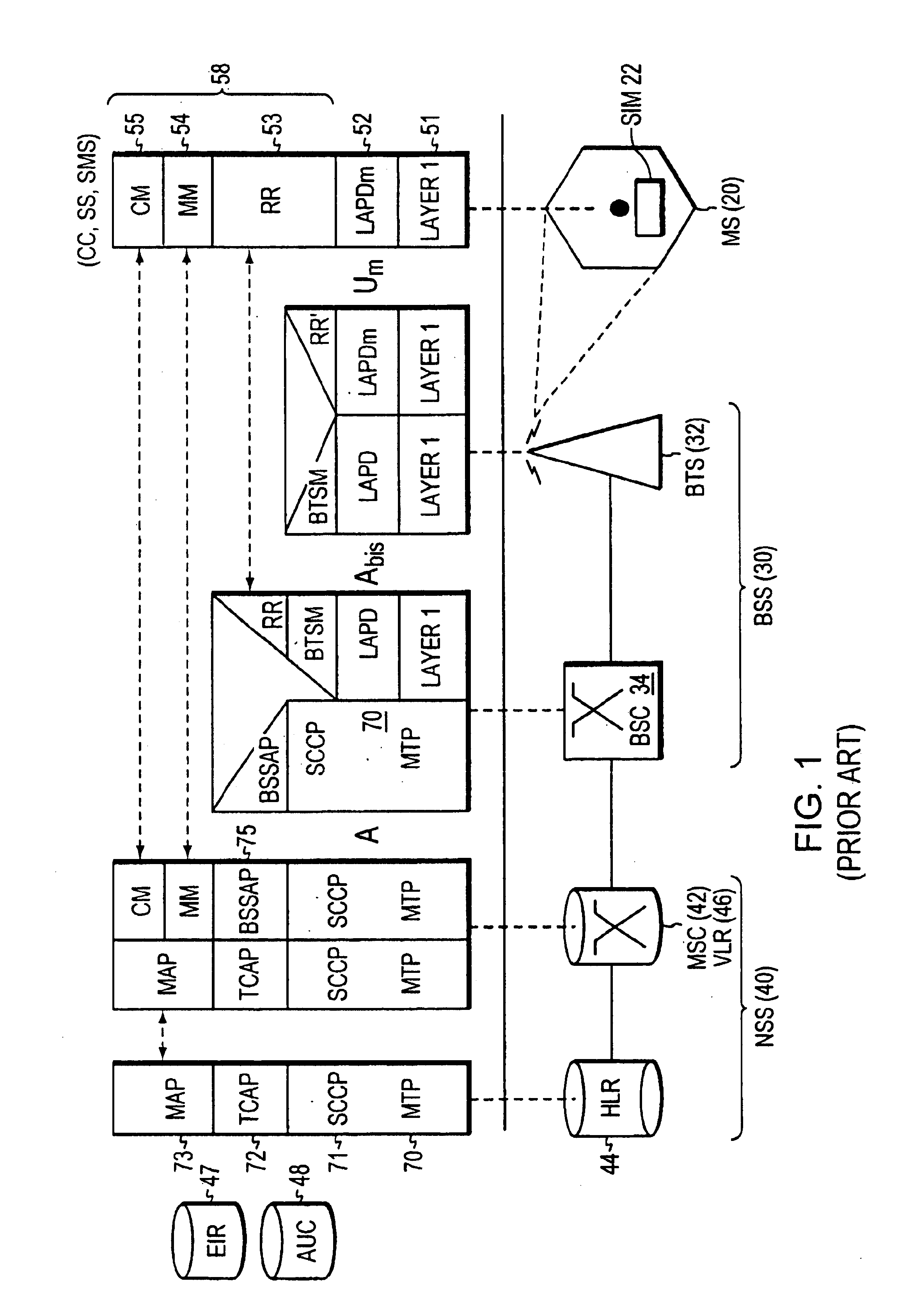
Quick layer-3 message multiplexing
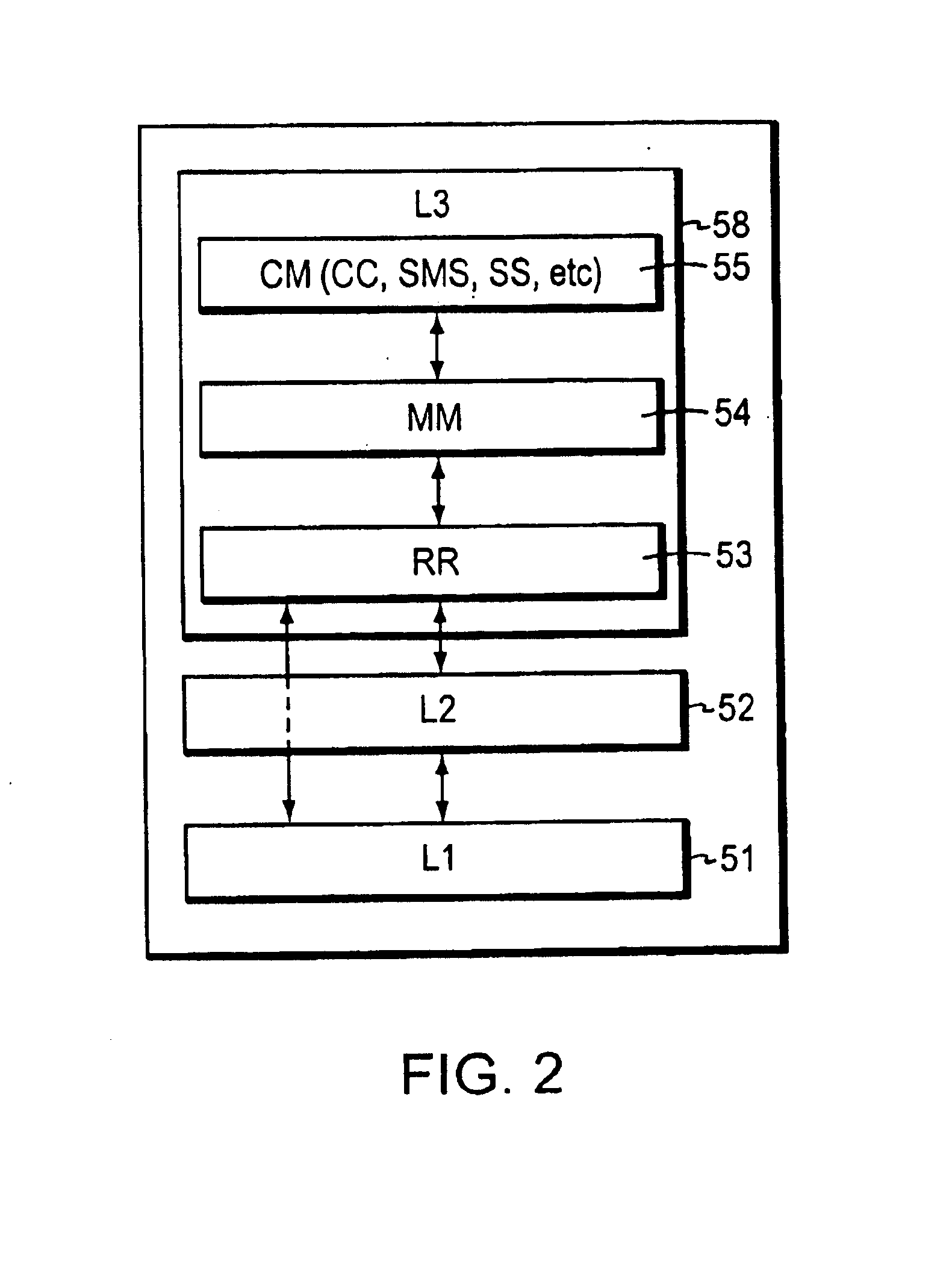
InactiveUS6879568B1Reduce processing timeReduce path delayInformation formatTime-division multiplexMultiplexingCommunications system
A technique for implementing a flattened Layer 3 stack model within a Global System for Mobile (GSM) communication system so that a centralized multiplex function associated with certain functions handles messages. The multiplex function dispatches radio resource (RR), mobility management (MM), or Connection Management (CM) function messages directly to the respective functional layers without first requiring such messages to pass through a stack. In the preferred arrangement, the multiplex function sub-L3 handles only uplink messages, allowing downlink messages to travel through the sub-layer stack without employing any bridging entity. The multiplex function can run independently of any of the other functions in Layer 3 or can be implemented as part of the message passing part of the Layer 2 running body so that the Layer 2 messages are routed directly to a respective RR, MM, or CM function. The flattened protocol stack permits time-sensitive messages related to location update, handover, or cell reselection and other time-critical messages to be handled more efficiently.
Owner:CISCO TECH INC
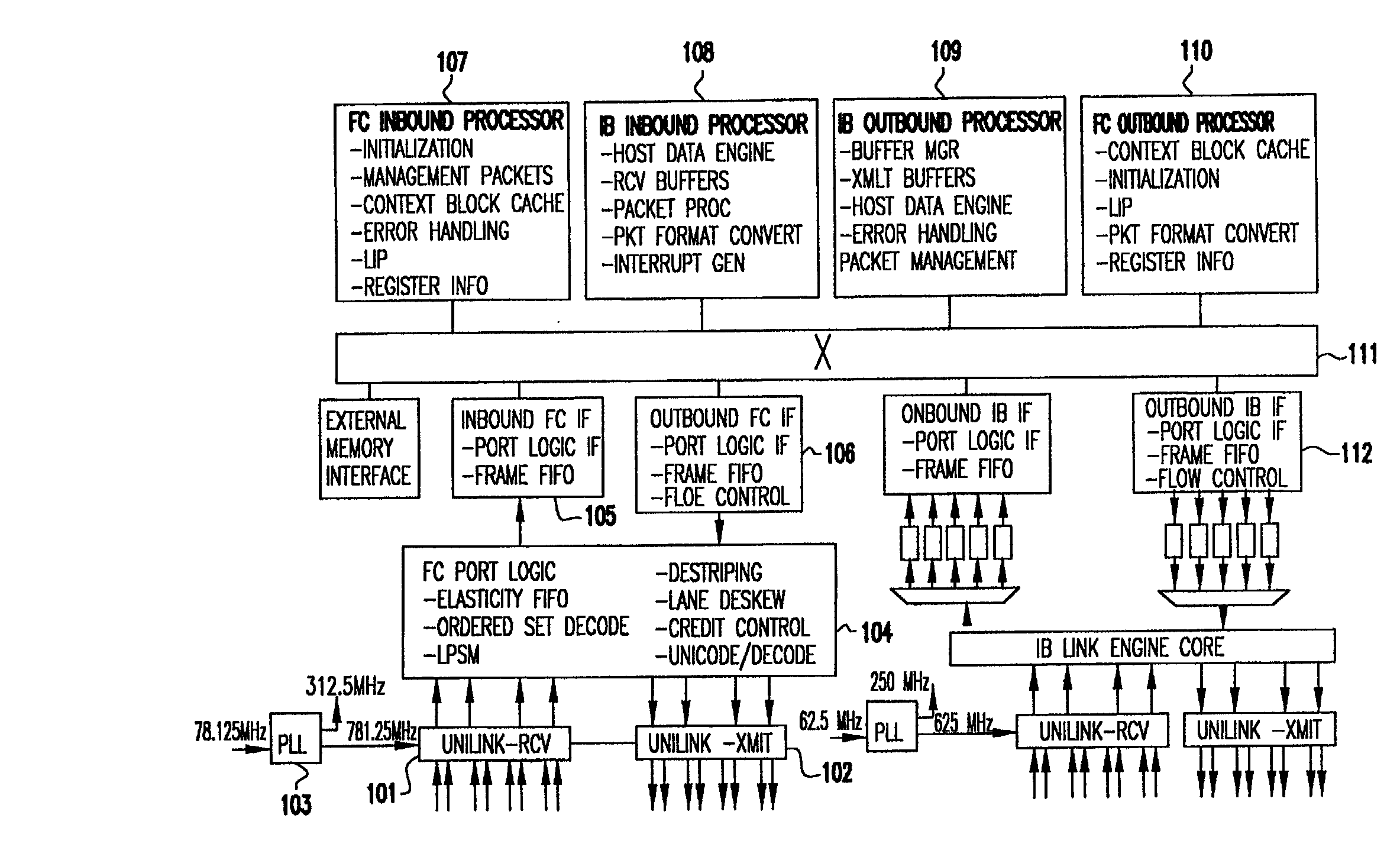
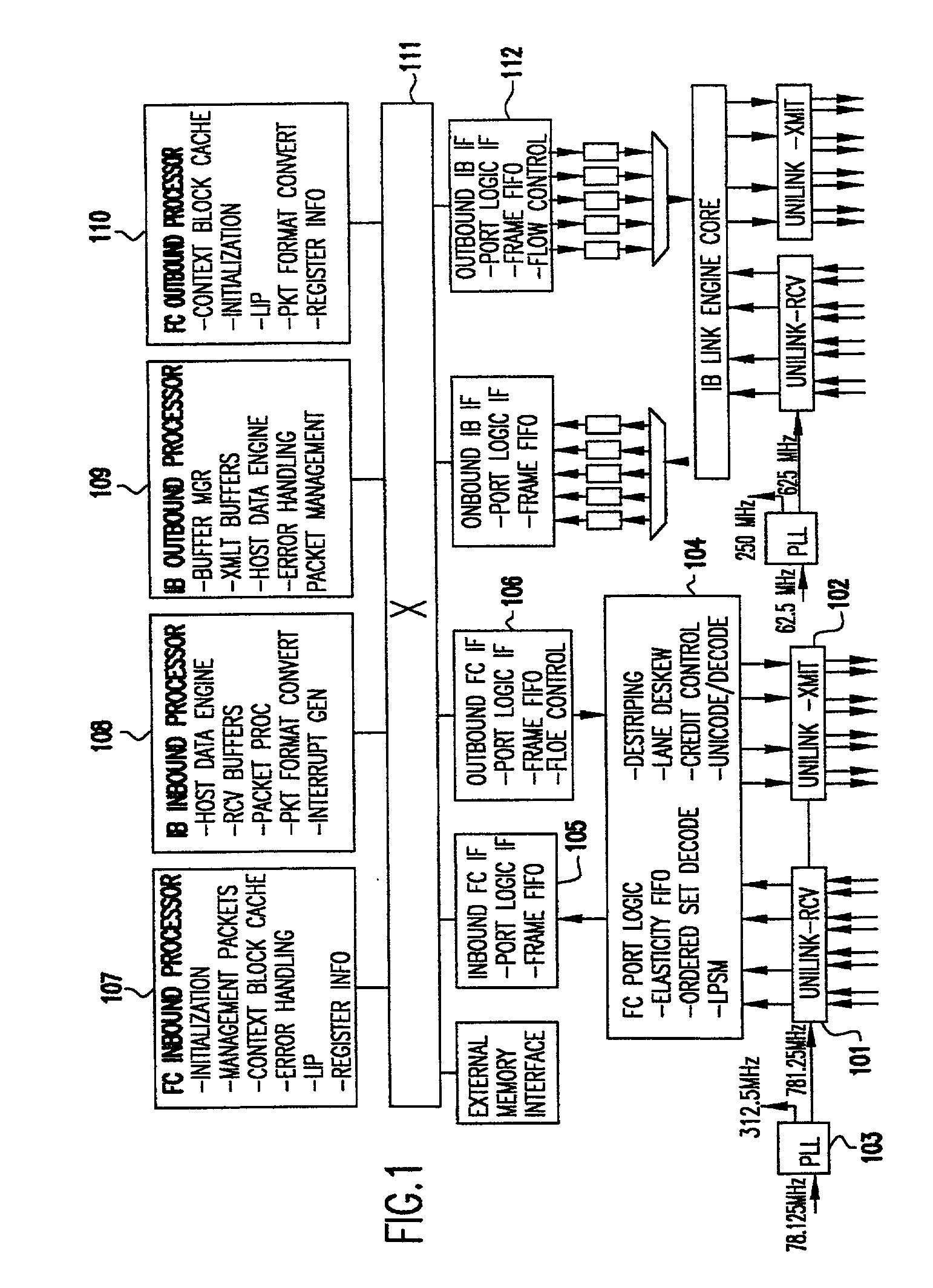
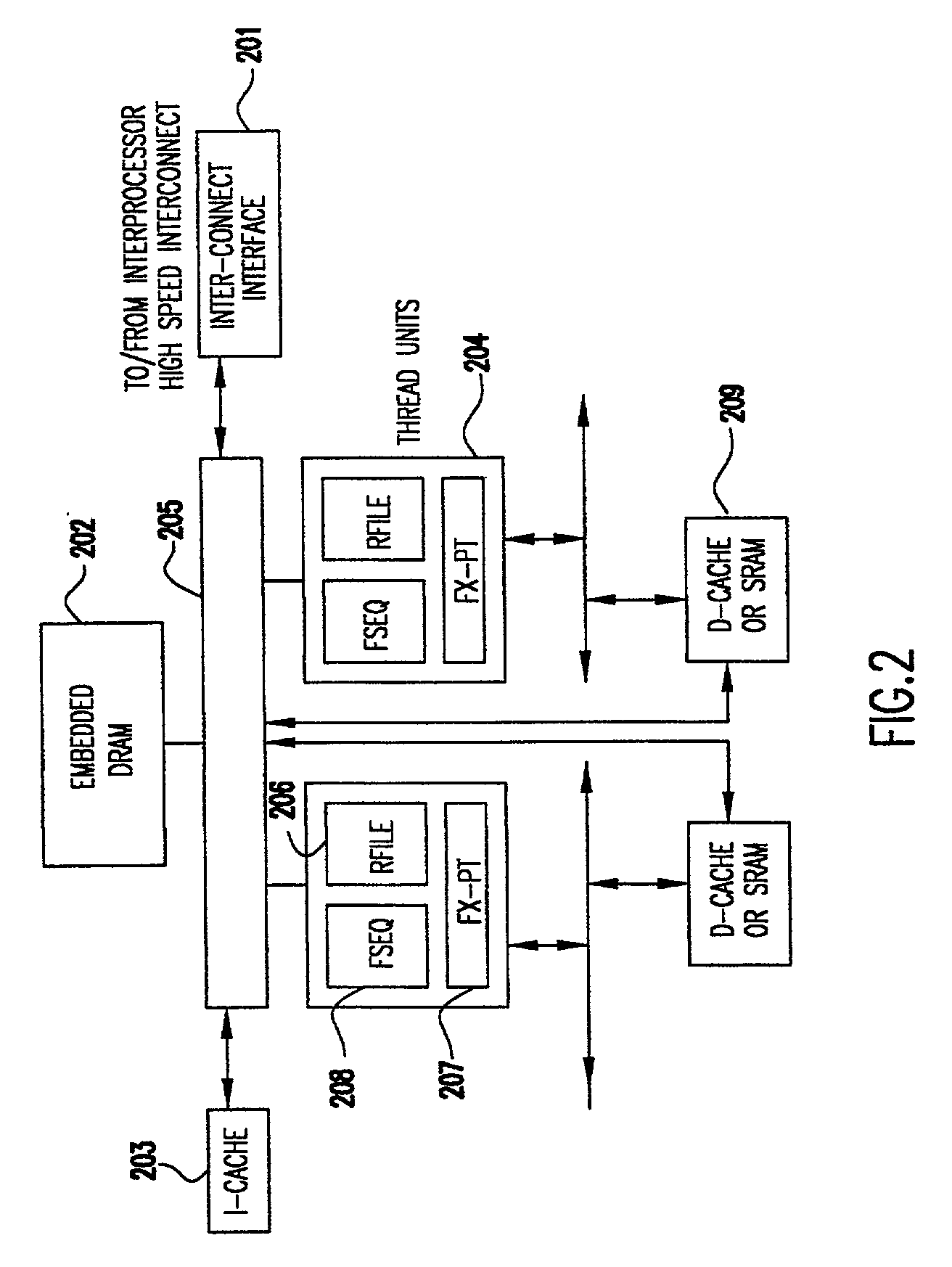
Programmable storage network protocol handler architecture
InactiveUS20030067913A1Resource allocationTime-division multiplexStatic random-access memoryProtocol processing
An architecture that achieves high speed performance in a network protocol handler combines parallelism and pipelining in multiple programmable processors, along with specialized front-end logic at the network interface that handles time critical protocol operations. The multiple processors are interconnected via high-speed interconnect, and each processor's memory is globally accessible by other processors. Each processor has multiple threads, each capable of fully executing programs. Each processor contains embedded dynamic random access memory (DRAM). Threads within a processor are assigned the processing of various protocol functions in a parallel / pipelined fashion. Data frame processing is done by one or more of the threads to identify related frames. Related frames are dispatched to the same thread so as to minimize the overhead associated with memory accesses and general protocol processing. The high-speed protocol handler may also provide built-in monitors for examining the activity of its hardware resources and reallocating the workload to the resources that are not heavily used, thus balancing the resource utilization and increasing the workload throughput.
Owner:IBM CORP
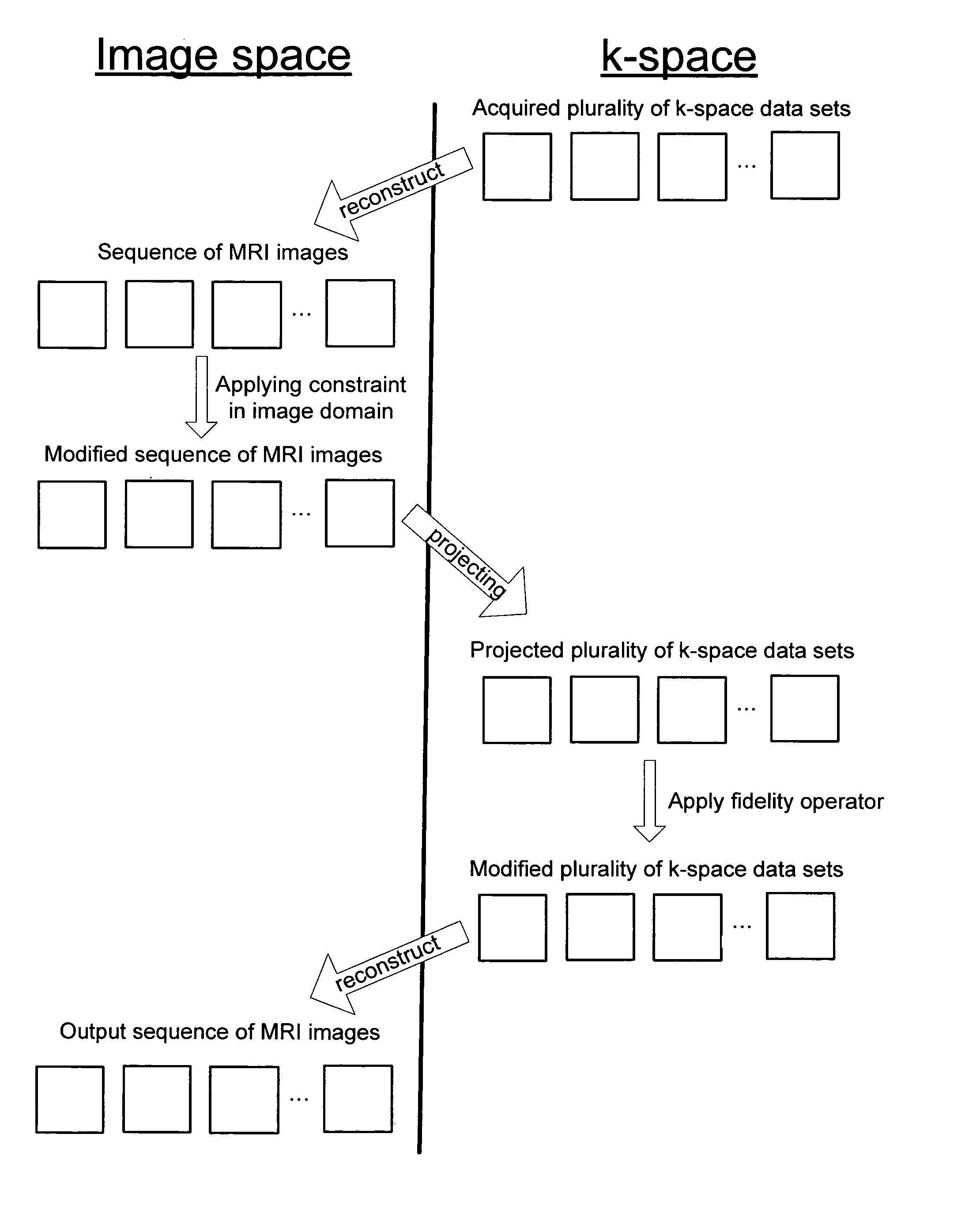
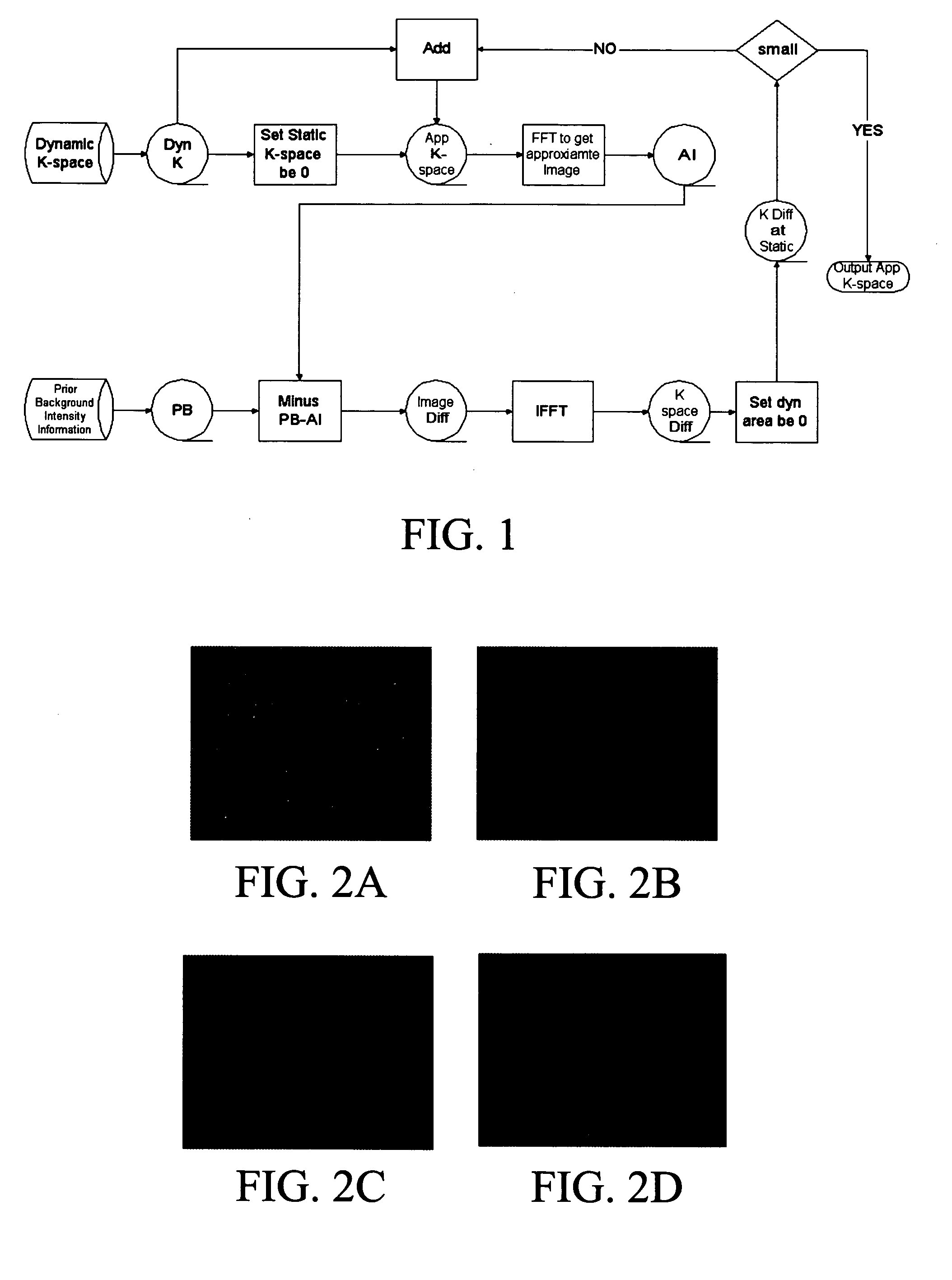
Method for generating fast magnetic resonance images
InactiveUS20050100202A1Shorten the timeQuality improvementMagnetic measurementsCharacter and pattern recognitionResonanceTime critical
The subject invention pertains to a method for acquiring and reconstructing a collection of time crucial magnetic resonance images. The subject invention is applicable for speeding up acquisition of or improving the quality of the set of images. In one specific embodiment, the subject method is used to reduce the time required to generate a cardiac CINE sequence of phases of the heart.
Owner:INVIVO CORP
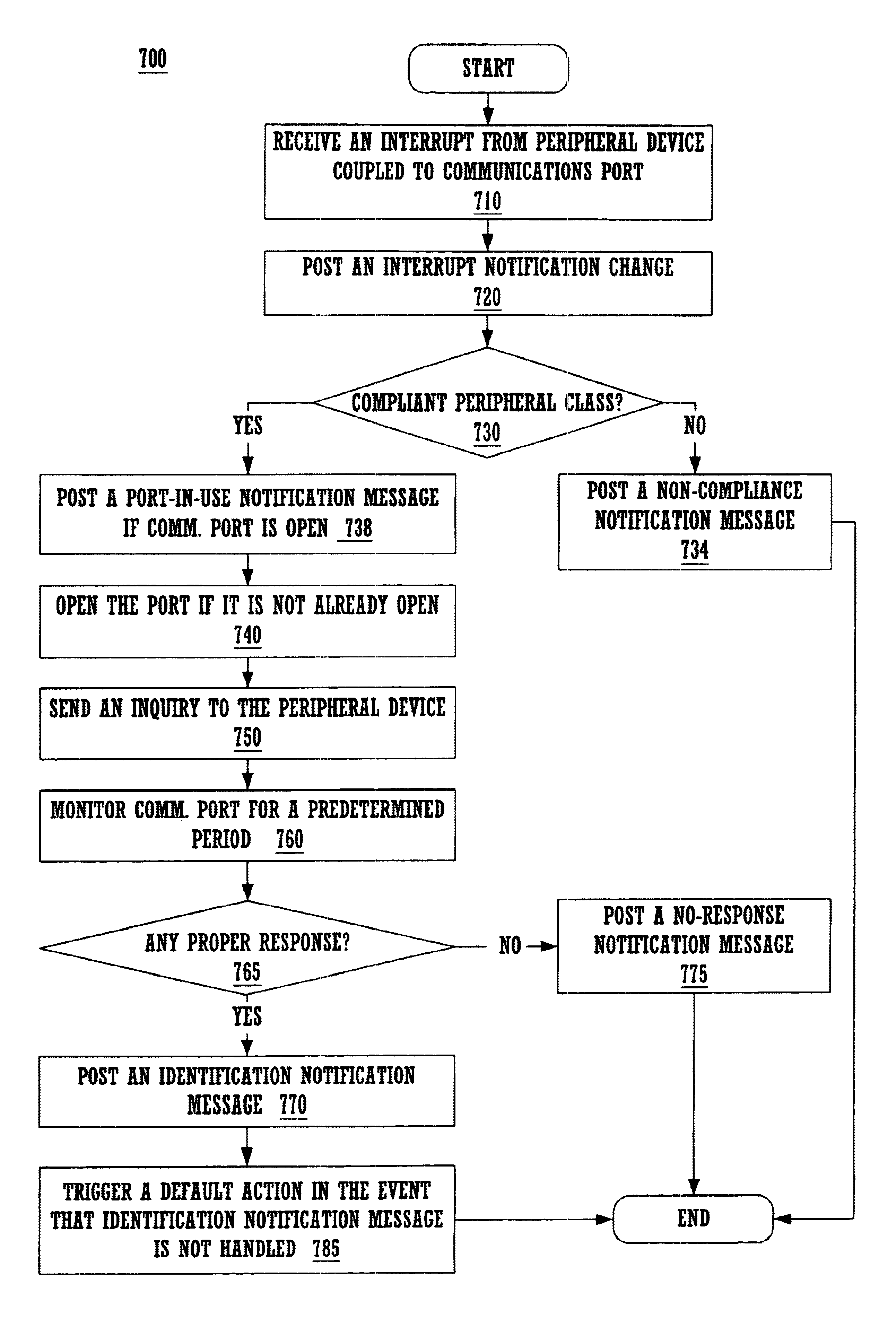
System for indentifying a peripheral device by sending an inquiry thereto after receiving an interrupt notification message if the interrupt and communication port meet predetermined conditions
Method and system for latency-independent peripheral device identification. In one embodiment, a computer system receives an interrupt from a peripheral device coupled to a computer system communications port. In response, an interrupt notification message is posted alerting a notification handler running on the system. It is determined whether the interrupt is indicates peripheral class compliance. In one embodiment, communications port device sense pin voltage is determinative. If the interrupt indicates peripheral class compliance and the communications port is inactive, the port is opened, and inquiry sent to the peripheral device via the open port. The computer system then waits for response from the peripheral device. If response is received within a predetermined time, identification is posted based on the response, including peripheral device classification information, so that a software handler registered with the operating system can handle the identification message when received. Thus, this embodiment imposes no time-critical interrupt response.
Owner:QUALCOMM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com