Method for inhibiting titanium dioxide phase change
A technology of titanium dioxide and phase transformation, applied in the field of inhibiting phase transformation of titanium dioxide, to achieve the effects of inhibiting phase transformation of titanium dioxide, simple operation and lowering temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
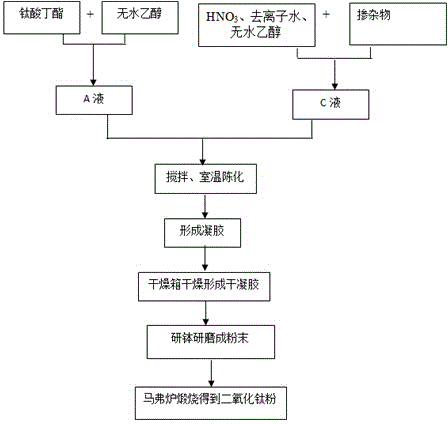
[0026] (1) According to the volume ratio of the precursor tetrabutyl titanate and absolute ethanol as 1:2, mix the precursor tetrabutyl titanate and absolute ethanol as liquid A;
[0027] (2) Press HNO 3 (mass percentage concentration is 65%), the volume ratio of deionized water and absolute ethanol is 1:3:17 and HNO 3 , deionized water, and absolute ethanol are mixed as liquid B;
[0028] (3) In a magnetic stirrer, slowly drop liquid B into liquid A, the pH value is 5, and then continue to stir for 1 hour;
[0029] (4) The sol was aged at room temperature for 24 hours, and then dried in a drying oven at 70°C for 96 hours;
[0030] (5) Take out the xerogel and grind it in an agate mortar;
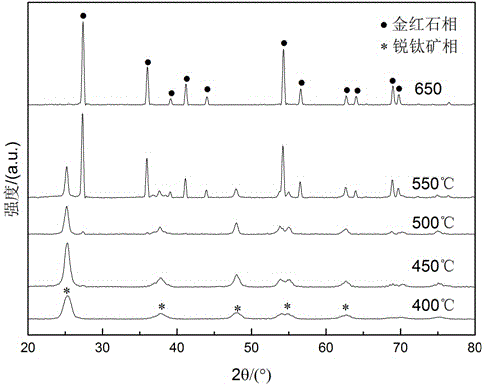
[0031] (6) Calcinate the powder obtained in step (6) at 400°C, 450°C, 550°C, and 650°C for 30 minutes, and then cool with the furnace to obtain pure titanium dioxide powder.
[0032] Such as figure 2 As shown in Table 1, 88.4 wt% of the pure titanium dioxide powder prepared by the pre...
Embodiment 2
[0034] (1) According to the volume ratio of the precursor tetrabutyl titanate and absolute ethanol as 1:3, mix the precursor tetrabutyl titanate and absolute ethanol as liquid A;
[0035] (2) Press HNO 3 (mass percentage concentration is 66%), the volume ratio of deionized water and absolute ethanol is 1:4:18 and HNO 3 , deionized water, and absolute ethanol are mixed as liquid B;
[0036] (3) Add an appropriate amount of Ce(NO 3 ) 3 9H 2 O is dissolved in B liquid;
[0037](4) In a magnetic stirrer, slowly drop liquid B into liquid A and the pH value is 4, then continue to stir for 1 hour;
[0038] (5) The sol was aged at room temperature for 48 hours, and then dried in a drying oven at 80°C for 72 hours;
[0039] (6) Take out the dry gel and grind it in an agate mortar;
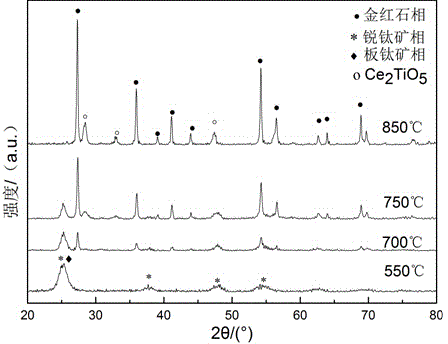
[0040] (7) The powder obtained in step (6) was calcined at 550°C, 700°C, 750°C, and 850°C for 30 minutes respectively, and then cooled in the furnace to obtain cerium-doped titanium dioxide powder. ...
Embodiment 3
[0043] (1) According to the volume ratio of the precursor tetrabutyl titanate and absolute ethanol as 1:4, mix the precursor tetrabutyl titanate and absolute ethanol as liquid A;
[0044] (2) Press HNO 3 (mass percentage concentration is 67%), the volume ratio of deionized water and absolute ethanol is 1:5:19 and HNO 3 , deionized water, and absolute ethanol are mixed as liquid B;
[0045] (3) Add appropriate amount of La 2 o 3 Soluble in liquid B;
[0046] (4) In a magnetic stirrer, slowly drop liquid B into liquid A and the pH value is 3, then continue to stir for 1 hour;
[0047] (5) The sol was aged at room temperature for 72 hours, and then dried in a drying oven at 100°C for 24 hours;
[0048] (6) Take out the dry gel and grind it in an agate mortar;
[0049] (7) The powder obtained in step (6) was calcined at 550°C, 650°C, 750°C, and 850°C for 30 minutes respectively, and then cooled in the furnace to obtain lanthanum-doped titanium dioxide powder.
[0050] Such ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com