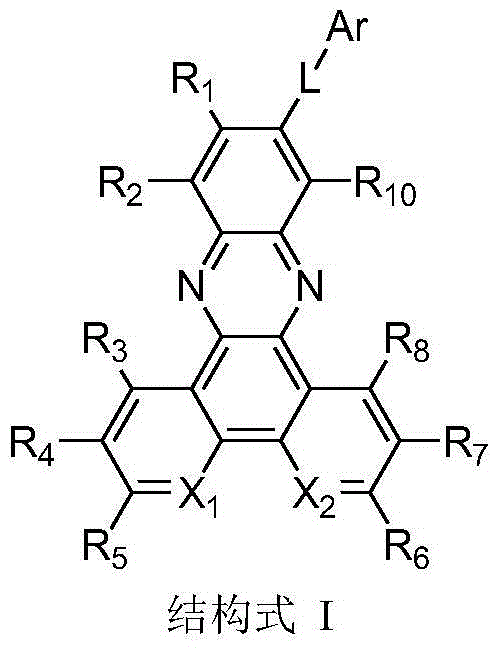
Organic electroluminescent compound of phenazine derivative
A technology of derivatives and compounds, applied in the field of organic electroluminescence, which can solve problems such as low electron mobility, low electron mobility, and voltage drop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
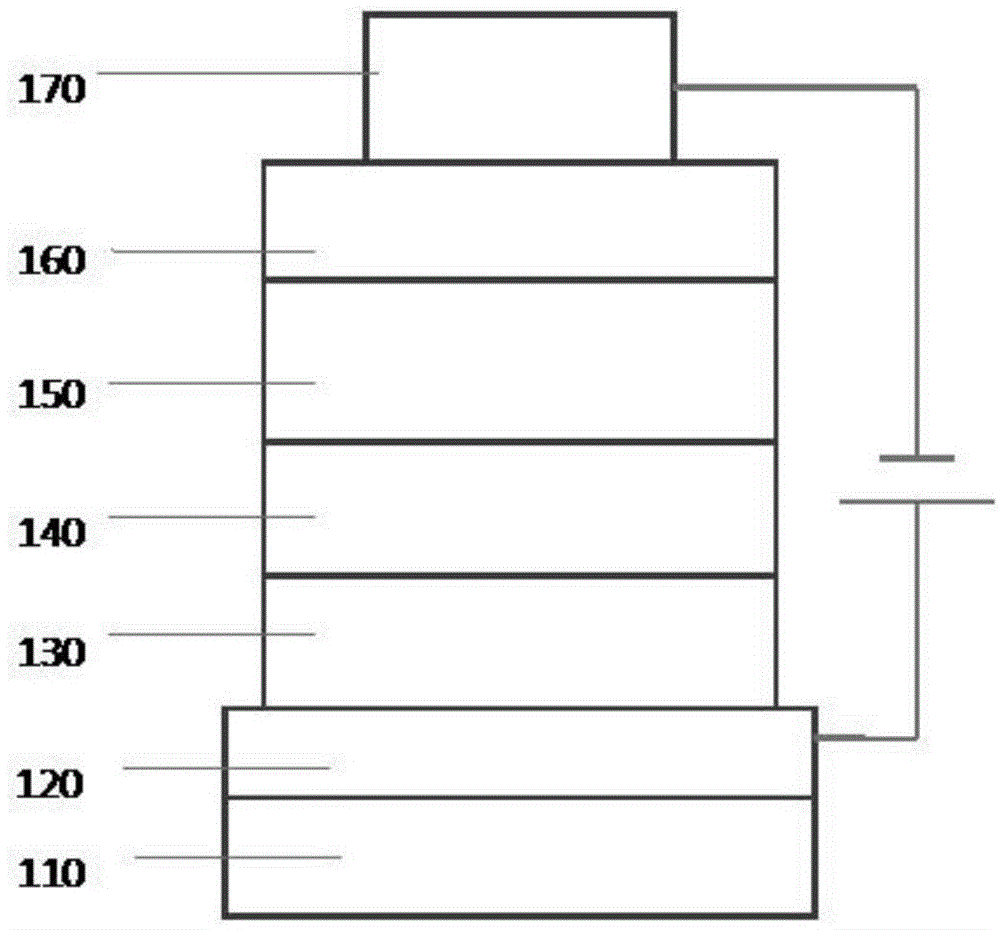
Image
Examples
Embodiment 1
[0074] Synthesis of compound 1
[0075]
[0076] Synthesis of Intermediate 1-1
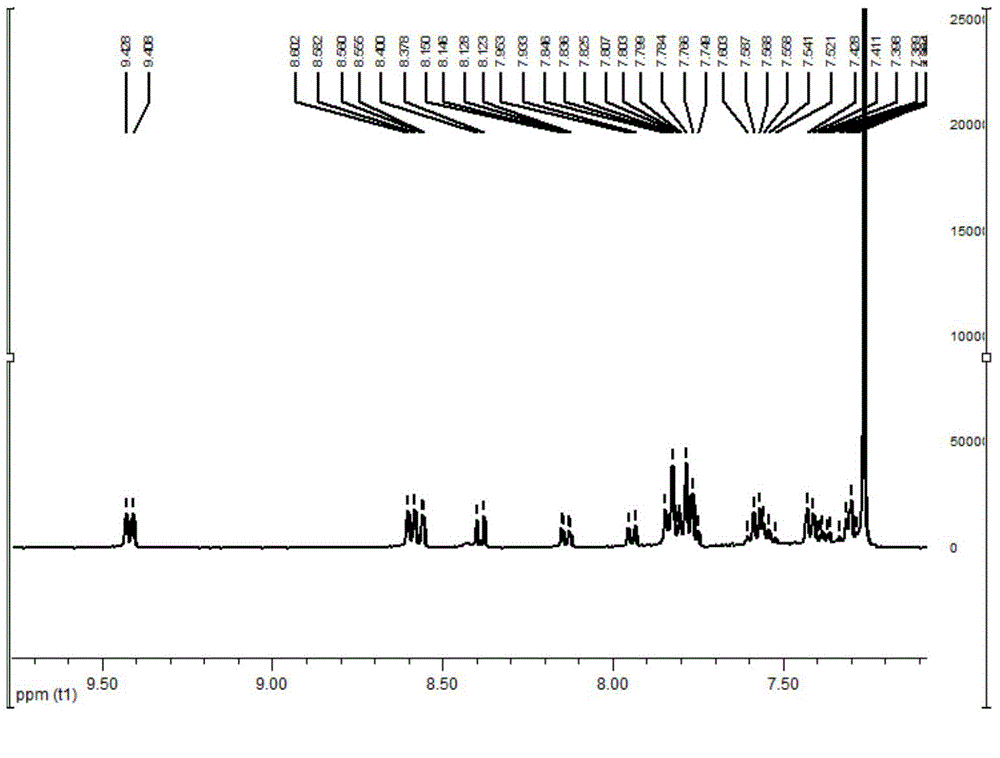
[0077] In a dry 100ml single-necked bottle, add phenanthrenequinone (2g, 9.6mmol), 4-bromo-o-phenylenediamine (1.8g, 9.6mmol), 50ml of absolute ethanol, and 25ml of acetic acid in sequence. After stirring at room temperature for 0.5 h, it was filtered to obtain 2.3 g of light yellow solid with a yield of 67%. 1 H NMR (400MHz, CDCl 3 )δ: 9.36(d, J=7.9Hz, 2H), 8.57(d, J=8.0Hz, 2H), 8.52(d, J=2.1Hz, 1H), 8.19(d, J=9.0Hz, 1H) ,7.91(dd,J=9.0,2.1Hz,1H),7.82(t,J=7.5Hz,2H),7.75(t,J=7.5Hz,2H).
[0078] Synthesis of compound 1
[0079] In a dry 50ml single-necked bottle, sequentially add intermediate 1-1 (0.3g, 8.4mmol), 4-(1-phenyl-1H-benzimidazol-2-yl)phenylboronic acid (0.27g, 8.4mmol) , K 2 CO 3 (2.3g, 16.8mmol), THF (30ml), water (5ml) and tetrakistriphenylphosphine palladium (50mg, 0.05mmol), under the protection of argon, the reaction solution was heated to reflux and stirred for 3h. After...
Embodiment 2
[0081] Synthesis of compound 17
[0082]
[0083] Synthesis of Intermediate 17-1
[0084] The synthesis method was similar to the synthesis of intermediate 1-1, and finally a light green solid was obtained with a yield of 60%. 1 H NMR (400MHz, CDCl 3 )δ: 9.59 (ddd, J = 8.1, 5.0, 1.8Hz, 2H), 9.29 (dd, J = 4.4, 1.7Hz, 2H), 8.55 (d, J = 2.1Hz, 1H), 8.21 (d, J =9.0Hz,1H),7.98(dd,J=9.0,2.2Hz,1H),7.80(ddd,J=8.1,4.5,1.4Hz,2H).
[0085] Synthesis of compound 17
[0086] In a dry 50ml single-necked bottle, sequentially add intermediate 17-1 (0.3g, 0.84mmol), 4-(1-phenyl-1H-benzimidazol-2-yl)phenylboronic acid (0.27g, 0.84mmol) , K 2 CO 3 (2.3g, 16.8mmol), THF (30ml), water (5ml) and tetrakistriphenylphosphine palladium (50mg, 0.05mmol), under the protection of argon, the reaction solution was heated to reflux and stirred for 3h. After the reaction stopped, Return to room temperature, wash with water, extract with dichloromethane, combine the organic phases, spin dry, and sepa...
Embodiment 3
[0088] Synthesis of compound 47
[0089]
[0090] Synthesis of Intermediate 47-1
[0091]Add compound 2-(4-bromophenyl)-4,6-diphenyl-1,3,5-triazine (2g, 5.1mmol), biboronic acid pinacol ester (1.5g, 6.18mmol) in the flask ), potassium acetate (15.4mmol), Pd(dppf) 2 Cl 2 (100mg), 40ml of N,N-dimethylformamide, heated to 90°C for 12 hours under nitrogen protection, cooled, poured into water, extracted with dichloromethane, dried with anhydrous sodium sulfate, concentrated, the crude product was used Silica gel column purification afforded 1.5 g of white solid with a yield of 68%. 1 H NMR (400MHz, CDCl 3, δ): 8.75-8.80 (m, 6H), 8.00-8.02 (d, J=8Hz, 2H), 7.57-7.63 (m, 6H), 1.40 (s, 12H).
[0092] Synthesis of compound 47
[0093] In a dry 50ml single-necked bottle, sequentially add intermediate 47-1 (0.4g, 0.92mmol), intermediate 1-1 (0.33g, 0.92mmol), K 2 CO 3 (2.3g, 16.8mmol), THF (30ml), water (5ml) and tetrakistriphenylphosphine palladium (50mg, 0.05mmol), under the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com