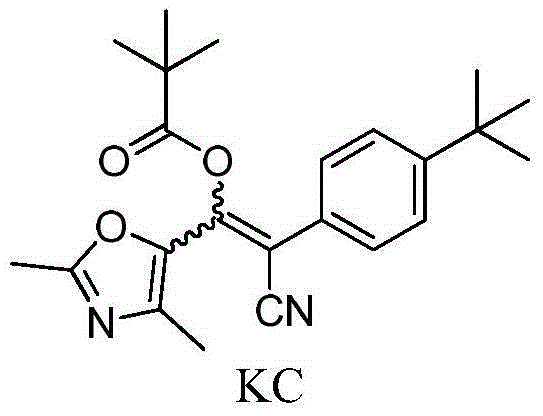
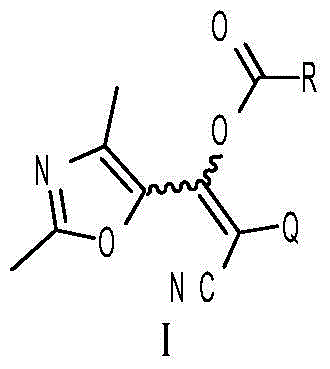
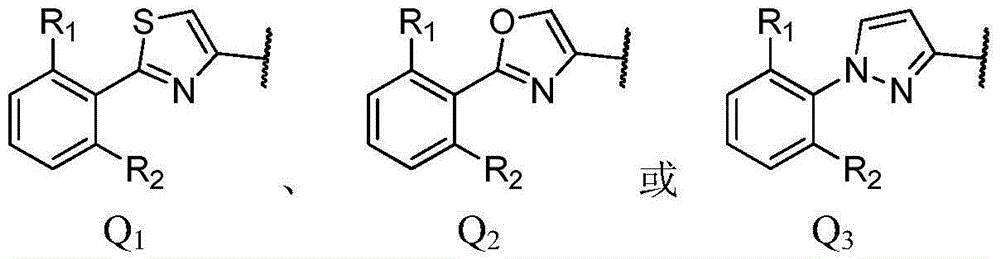
2,4-dimethyl oxazole acrylonitrile compound and application thereof
A compound and alkyl technology, applied in the field of insecticides and acaricides, can solve the problems that the insecticidal and acaricidal activities have not been disclosed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Embodiment 1, the preparation of compound 137
[0102] (1) Preparation of 2-bromothiobenzamide
[0103]
[0104] Add 2-bromobenzamide (50.00 g, 0.25 mol), Lawesson's reagent (109.10 g, 0.27 mol), and 200 ml of toluene into the three-necked flask, heat up to reflux, and react at reflux for 8 hours. After the reaction was completed, it was lowered to room temperature and filtered. The filtrate was washed with 2×200 ml of water, concentrated, and directly used in the next reaction without further purification.
[0105] (2) Preparation of 4-chloromethyl-2-(2-bromophenyl)thiazole
[0106]
[0107] Add the 2-bromothiobenzamide prepared in the previous step, 200 ml of methanol, and 1,3-dichloroacetone (34.30 g, 0.27 mol) into the three-necked flask, raise the temperature to reflux, and react under reflux for 16 hours. After the reaction was completed, it was lowered to room temperature and filtered. The filtrate was concentrated, and the residue was separated by colu...
Embodiment 2
[0117] Embodiment 2, the preparation of compound 375
[0118] (1) Preparation of 4-chloromethyl-2-(2-chlorophenyl)oxazole
[0119]
[0120] Add 2-chlorobenzamide (2.80 g, 0.018 mol) and 1,3-dichloroacetone (4.60 g, 0.036 mol) into a 100 ml round bottom flask, heat to melt, then continue to heat up to reflux and keep the reaction at 4 hours at reflux. After stopping the reaction, cool to room temperature naturally, pour into 50 ml of water, extract with 3 × 10 ml of ethyl acetate, wash the organic phase with 3 × 20 ml of saturated aqueous sodium chloride solution, dry with anhydrous magnesium sulfate, concentrate and column Chromatographic separation (eluent: ethyl acetate:petroleum ether=1:3) gave 3.10 g of 4-chloromethyl-2-(2-chlorophenyl)oxazole as a yellow solid with a yield of 77%.
[0121] (2) Preparation of 2-(2-chlorophenyl)-4-cyanomethyl oxazole
[0122]
[0123] Add 4-chloromethyl-2-(2-chlorophenyl) oxazole (45.50 g, 0.20 mol), 200 ml of dimethyl sulfoxide, s...
Embodiment 3
[0130] Embodiment 3, the preparation of compound 471
[0131] (1) 3-(2,4-dimethyloxazol-5-yl)-3-hydroxy-2-(2-(2,6-difluorophenyl)oxazol-4-yl)acrylonitrile preparation
[0132]
[0133] Under an ice-water bath, 2-(2-(2,6-difluorophenyl)oxazol-4-yl)acetonitrile (1.32 g, 0.006 mol), (2,4-dimethyloxa Azol-5-yl)(1H-pyrazol-1-yl)methanone (1.15 g, 0.006 mol), 20 ml of tetrahydrofuran, after stirring for about 30 minutes, potassium tert-butoxide (1.35 g, 0.012 mol) was added in batches After the addition, the reaction solution was warmed up to room temperature and reacted at room temperature for 2 hours. After stopping the reaction, the solvent in the system was distilled off under reduced pressure. After the residue was fully dissolved with 200 milliliters of water, the acid was adjusted to pH 2 to 3 with concentrated hydrochloric acid. Solids were precipitated in the system, filtered and dried to obtain 2.00 grams of 3-( 2,4-Dimethyloxazol-5-yl)-3-hydroxy-2-(2-(2,6-difluoroph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com