Application of using protein-coated iron-based magnetic nano-particle as magnetic hyperthermia agent
A magnetic nanoparticle and magnetic nanoparticle technology, applied in the medical application field of biological nanomaterials, can solve the problems of magnetic nanoparticle saturation magnetization value decrease, contribution reduction, weak magnetocaloric performance, etc., to achieve good biocompatibility, good Effects of colloidal dispersion stability and high magnetic hyperthermia performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
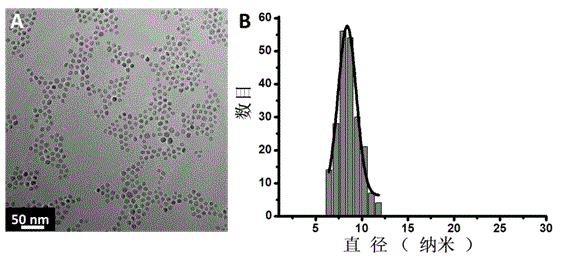
[0022] 9 nm Fe 3 o 4 Preparation of : Reference Journal of Materials Chemistry 2012, 22, 8235-8244.
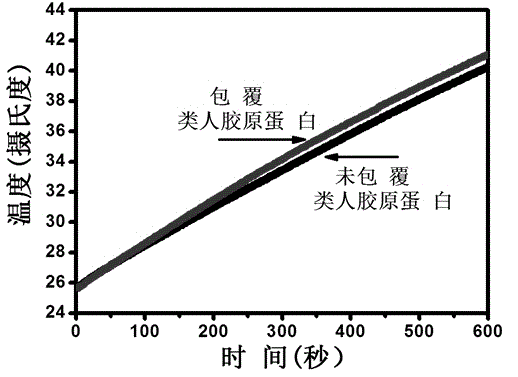
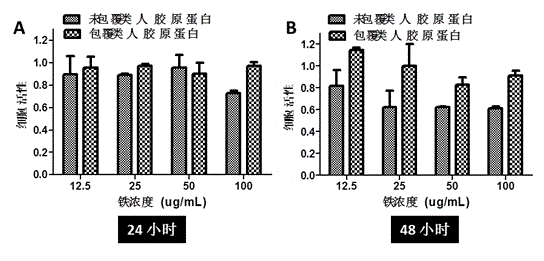
[0023] Preparation of human-like collagen: Human-like collagen is a new type of biomaterial with a molecular weight of 97 kDa. It is a polymer organism produced by reverse-transcribing the mRNA of human collagen into cDNA, recombining a section of the gene after digestion into E.coli (Escherichia coli), and producing it through high-density fermentation, separation, renaturation, and purification processes protein. Unlike collagen derived from animals, human-like collagen has low immunogenicity and is easily soluble in aqueous solutions. Moreover, human-like collagen has good biocompatibility and can be further used in tissue engineering. Refer to Chinese patent ZL01 106757.8 for a human-like collagen and its production method.
[0024] 9 nm Fe 3 o 4 Magnetic nanoparticles were dispersed in n-hexane to obtain Fe with a solid content of 20 mg / mL 3 o 4 n-hexane solution...
Embodiment 2
[0030] 17 nm Fe 3 o 4 Magnetic nanoparticles were dispersed in n-hexane to obtain Fe with a solid content of 15 mg / mL 3 o 4 hexane solution of magnetic nanoparticles, take 2 mL of the solution, add 10 mL of ultrapure water and 20 mg of cetyltrimethylammonium bromide (CTAB), ultrasonically stir for 20 min to form a microemulsion, add 0.037 mL NaClO solution, stirred for 50 min, then adjusted the pH value of the solution to 12.5, then added 0.25 mL NaClO solution and 0.6 mg RuCl 3 , continue to stir the reaction for 1 h. After the reaction is over, the Fe 3 o 4 The magnetic nanoparticles are separated from the solution, the supernatant solution is discarded, and the Fe 3 o 4 The magnetic nanoparticles were washed 3 times with ultrapure water and dispersed in ultrapure water to obtain aqueous phase dispersed Fe 3 o 4 magnetic nanoparticles.
[0031] Wash the above-mentioned aqueous phase magnetic microspheres three times with deionized water and magnetically separate the...
Embodiment 3
[0036] 15 nm Fe 3 o 4 Magnetic nanoparticles were dispersed in n-hexane to obtain Fe with a solid content of 20 mg / mL 3 o 4 hexane solution of magnetic nanoparticles, take 2 mL of the solution, add 10 mL of ultrapure water and 20 mg of cetyltrimethylammonium bromide (CTAB), ultrasonically stir for 20 min to form a microemulsion, add 0.037 mL NaClO solution, stirred for 50 min, then adjusted the pH value of the solution to 12.5, then added 0.25 mL NaClO solution and 0.6 mg RuCl 3 , continue to stir the reaction for 1 h. After the reaction is over, the Fe 3 o 4 The magnetic nanoparticles are separated from the solution, the supernatant solution is discarded, and the Fe 3 o 4 The magnetic nanoparticles were washed 3 times with ultrapure water and dispersed in ultrapure water to obtain aqueous phase dispersed Fe 3 o 4 magnetic nanoparticles.
[0037] Wash the above-mentioned aqueous phase magnetic microspheres three times with deionized water and magnetically separate the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com