Production of high-purity lithium difluorophosphate
A technology of lithium difluorophosphate and lithium fluoride, which is applied in the field of low-sodium lithium difluorophosphate, sodium or potassium ions, and can solve problems such as inability to meet purity requirements and complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
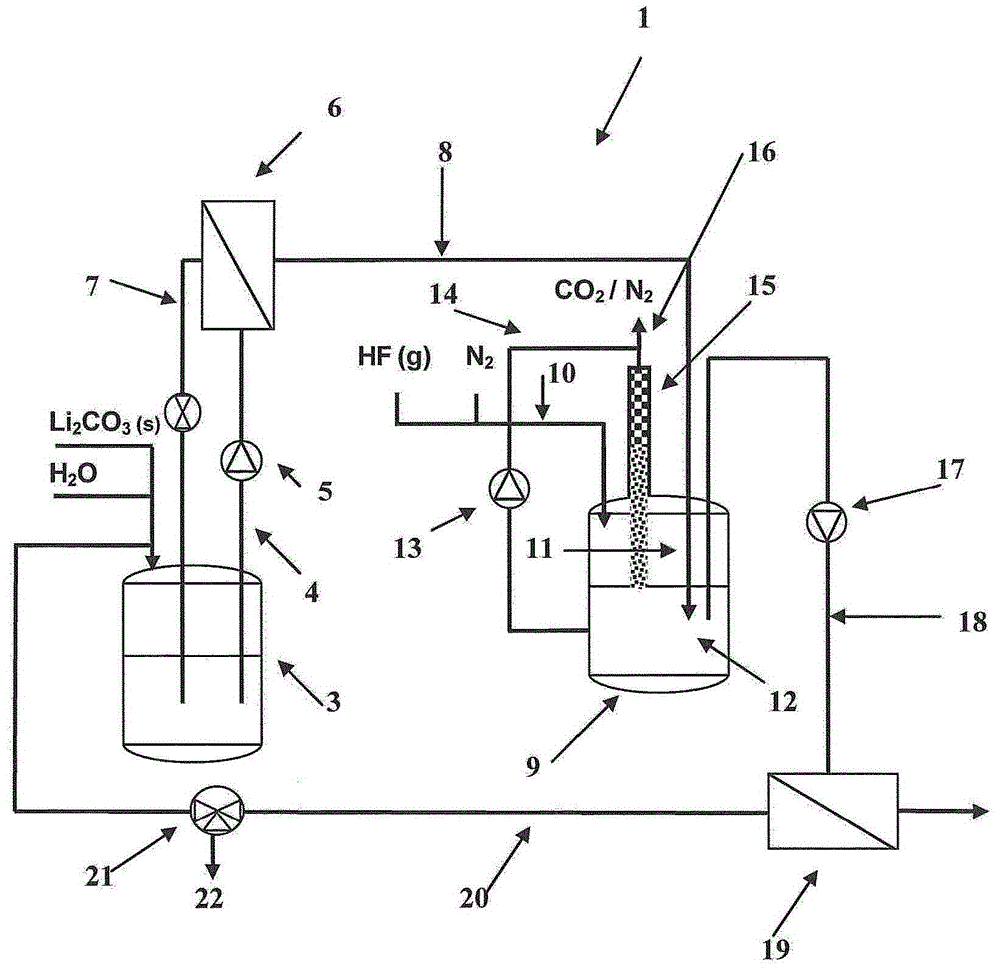
[0106] pass figure 1 The preparation of lithium fluoride will be described in detail.
[0107] In a device 1 for preparing lithium fluoride, water (H 2 O) makes solid lithium carbonate (Li 2 CO 3 (s)) suspend and, if the device 1 is not filled for the first time, the filtrate from the filter unit 19 in the reservoir 3, and the lithium carbonate at least partly go into solution. The suspension thus obtained is conveyed via line 4 by pump 5 to a filtration unit 6 (here in the form of a cross-flow filter), wherein undissolved lithium carbonate is recycled via line 7 to storage 3, and The filtrate (aqueous medium containing dissolved lithium carbonate) is introduced from line 8 into reactor 9 . In the reactor 9, a gas stream comprising gaseous hydrogen fluoride (which here comprises gaseous hydrogen fluoride and nitrogen) is introduced via line 10 into the gas space 11 of the reactor (which is above the liquid space 12 of the reactor) . A pump 13 directs the contents of the ...
example
[0191] The unit "%" is to be understood hereinafter always as meaning % by weight.
[0192] Regarding the ion chromatography method used in the course of this study, reference is made to a publication of the TU Bergakademie Freiberg, Faculty of Chemistry and Physics, Department of Analytical Chemistry, March 2002 , and the literature cited therein.
[0193] During the course of this study, LiPO 2 f 2 The concentrations were measured using an ion chromatograph with the following parameters:
[0194] Instrument type: Dionex ICS 2100
[0195] column: AS202*250-mm "analytical column with guard"
[0196] Sample volume: 1μl
[0197] Eluent: KOH Gradient: 0min / 15mM, 10min / 15mM, 13min / 80mM, 27min / 100mM, 27.1min / 15mM, 34min / 15mM
[0198] Eluent flow rate: 0.25ml / min
[0199] Temperature: 30°C
[0200] Self-regenerating suppressors: 300(2-mm)
[0201] For LiPO 2 f 2 The obtained signal is specified by 31 P and 19 Confirmed by FNMR spectroscopy. Separation of LiPO from ...
example 1
[0202] Example 1: Preparation of high-purity lithium fluoride
[0203] on a basis figure 1 In the device, the reservoir 3 is initially loaded with 500 g of industrial grade quality (purity: >98% by weight; Na: 231 ppm, K: 98 ppm, Mg: 66 ppm, Ca: 239 ppm) of solid lithium carbonate and 20 l of water, and prepare a suspension at 20°C. After about five minutes, the suspension is directed to a filtration unit 6 (which takes the form of a cross-flow filter), and the resulting medium comprising dissolved lithium carbonate (here a Aqueous solution of lithium carbonate) is led into reactor 9 via line 8.
[0204] After a total of 4 kg of medium has been pumped into the reactor 9, the feed from the filter unit 6 is stopped and the feed of gaseous hydrogen fluoride into the gas space 11 is started in this reactor 9 via pump 13, line 14 and A column 15 with random packing circulates the medium continuously with pumping. This metering was ended when the pH of the solution circulated by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com