A class of pentapeptide analogues of insect depressantin and its application
A technology of analogs and side voxels, which is applied in the field of cockroach control, can solve the problems of complex structure, high synthesis cost, complex structure, etc., and achieve the effect of inhibiting synthesis and egg development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The compound of formula A of the present invention is prepared according to the method of polypeptide solid-phase synthesis.
[0033] Taking compound A1 as an example, its preparation method is as follows:
[0034] (1) Resin activation: Weigh 220 mg Rink Amide-AM resin, swell and activate it with 15 ml DCM for 3 h, add 20% piperidine in DMF solution, cut for 20 min, wash with DMF and DCM 4 times, and monitor the reaction with Kaiser’s reagent.
[0035] (2) Connecting to Leu: add Fmoc-Leu-OH (3 times the amount), HBTU (3 times the amount), HOBt (3 times the amount), DIEA (6 times the amount), dissolve in 15ml DMF, stir at room temperature for 2 hours, and remove the reaction Solution, DMF and DCM were washed 4 times each, and Kaiser's reagent was used to monitor the reaction. Then add 20% piperidine DMF solution to cut for 20min, and wash 4 times with DMF.
[0036] (3) Gly connection: add Fmoc-Gly-OH (3 times the amount), HBTU (3 times the amount), HOBt (3 times the am...
Embodiment 2
[0048] The in vitro activity of embodiment 2 compound
[0049] The present invention adopts the in vitro radiochemical assay method of Tobe et al. -179.b.Tobe, S.S.; Pratt, G.E.The influence of substrate concentrations on the rate of insect juvenile hormone biosynthesis by corpora allata of thedesert locust in vitro.Biochem.J.1974,144,107-113.), determined the formula A compound In vitro activity to inhibit juvenile hormone synthesis in Pacific cockroach (Diploptera punctata). The in vitro bioassay results of the compound of formula A are shown in Table 2.
[0050] Table 2 The in vitro activity of the compound of formula A
[0051]
[0052]
[0053] Table 2 result shows: formula A compound can obviously inhibit the synthesis of Pacific cockroach (Diplopterapunctata) juvenile hormone, and the in vitro activity of A1-A8 is better than core pentapeptide, and wherein the in vitro activity of analogue A6 is core pentapeptide 103 times.
Embodiment 3
[0054] Embodiment 3 Injection test compound's in vivo activity
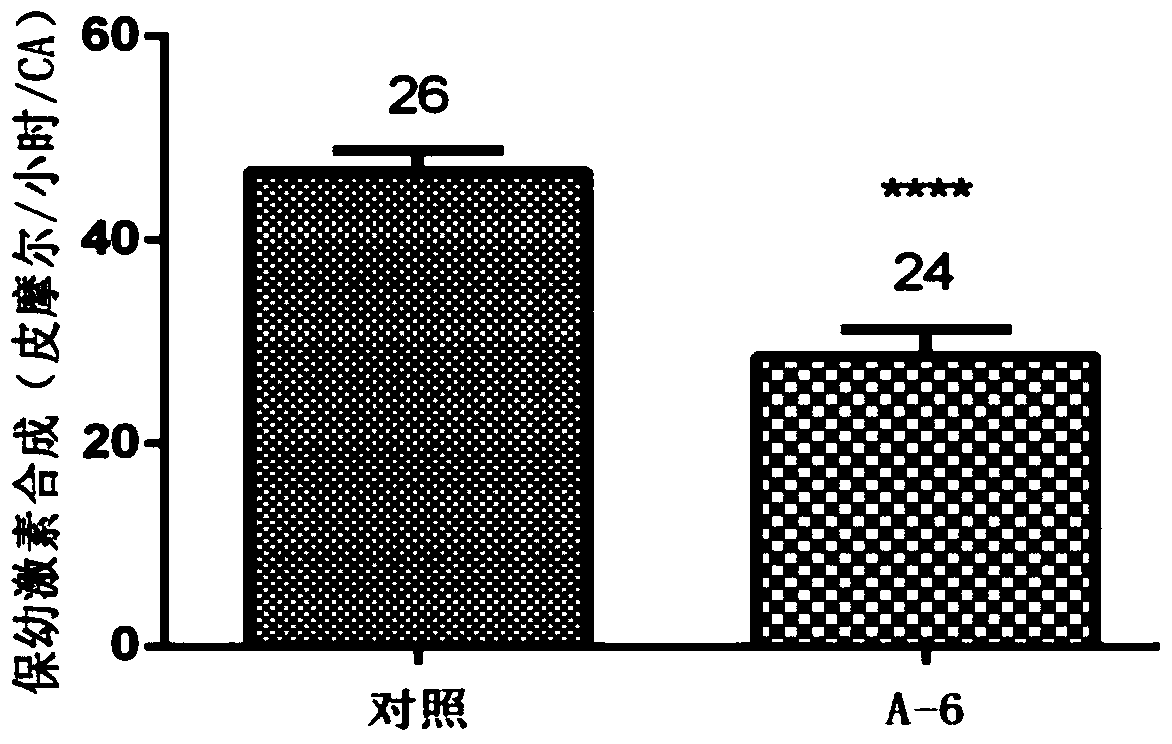
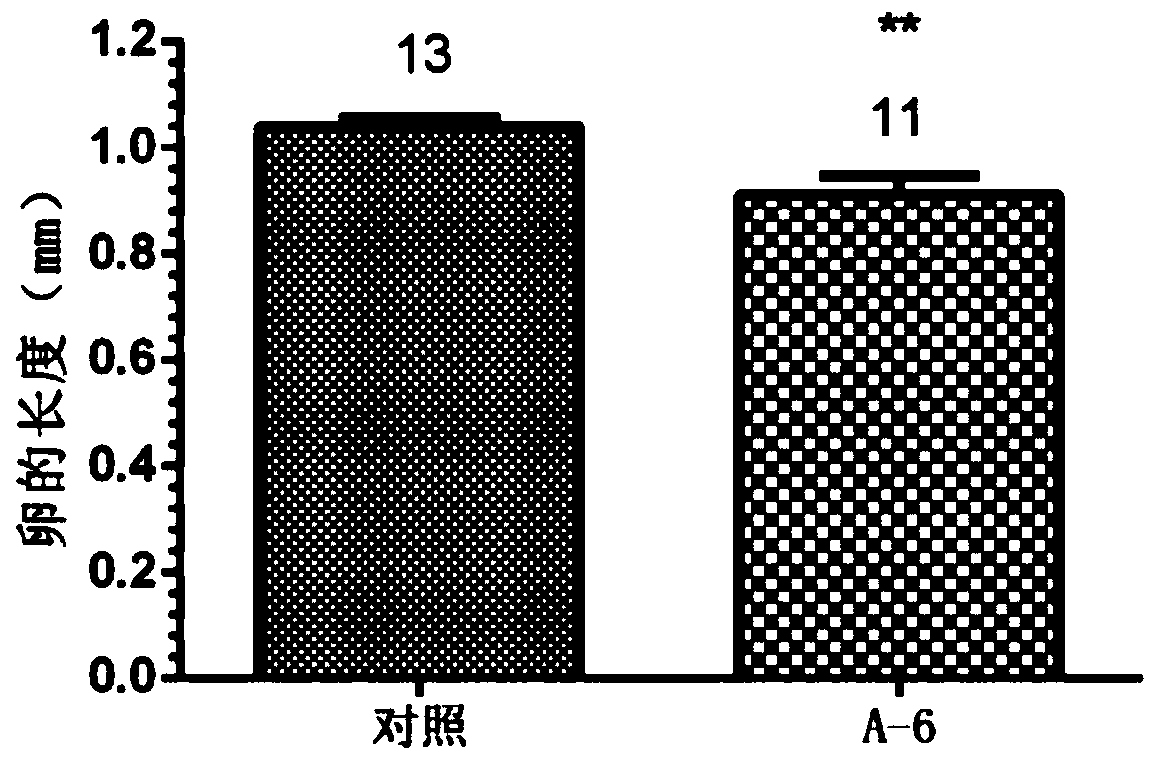
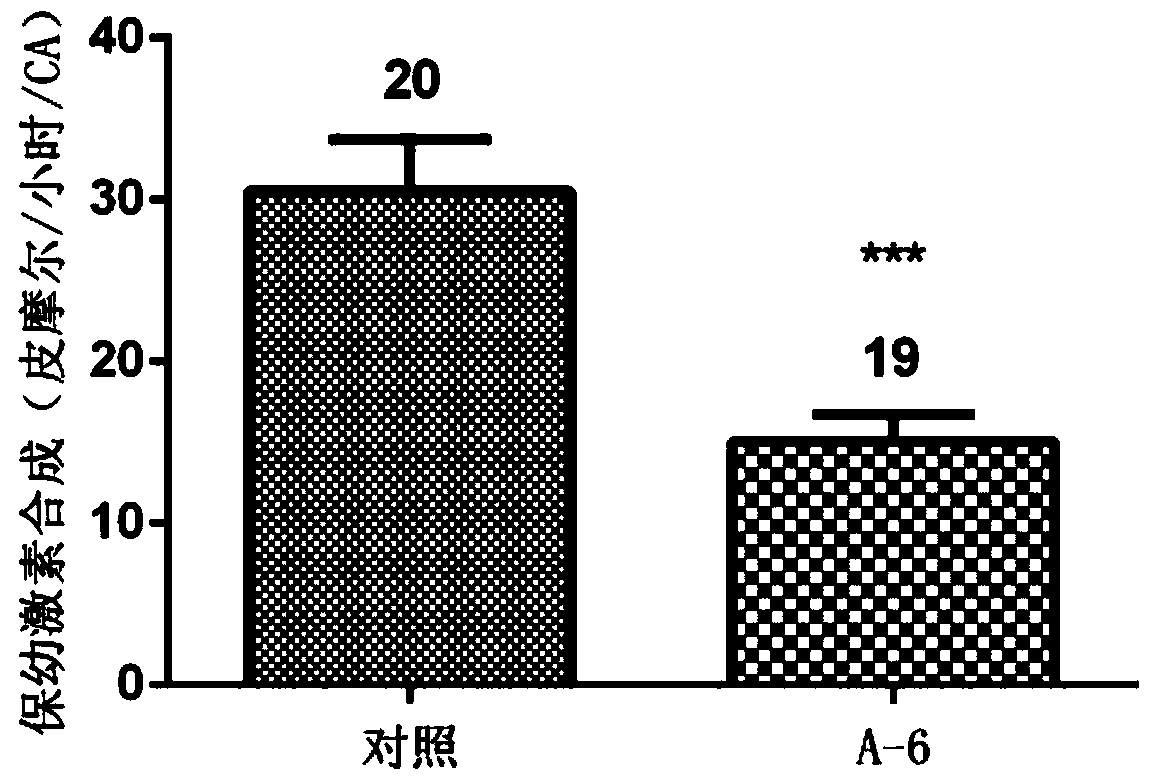
[0055] Prepare a 10 μM aqueous solution of Compound A6, select female cockroaches on the first day after adult eclosion, inject 5 μL at the joints of the legs, place the injected cockroaches at 27±1°C, humidity 80%, sufficient food and water, and no light After cultured until the third day, the ability of the injected cockroaches to synthesize juvenile hormone was tested by the method of in vitro radiochemical assay, and the length of the eggs was measured.
[0056] The results of compound A6 inhibiting the synthesis of juvenile hormone and inhibiting egg development are as follows: figure 1 , figure 2 shown. The results of the injection test showed that the compound A6 had a significant inhibitory effect on the biosynthesis of cockroach juvenile hormone, and also had a significant inhibitory effect on the development of its eggs. All experimental results are statistically analyzed by t test method, **** mean...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com