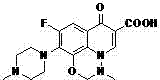
A kind of Mabaofloxacin microcapsule and preparation method thereof
A technology of mabrofloxacin and solution, applied in the field of mabrofloxacin microcapsules and preparation thereof, can solve the problems of low solubility, restrict the development and application of mabrofloxacin preparations, etc. The effect of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Starch Sodium Octenylsuccinate 40%
[0023] Mabofloxacin 60%
[0024] Preparation:
[0025] Dissolve starch sodium octenyl succinate in purified water to prepare a wall material solution with a concentration of 10%. Dissolve Mabofloxacin in a mixed solution of dichloromethane / absolute ethanol (9:1 ratio) to prepare a core material solution with a concentration of 20%. At a shear rate of 5000r / min, slowly drop the core material solution into the wall material solution, and at the same time carry out vacuum distillation to recover the mixed solution of dichloromethane / absolute ethanol. The system temperature is 10°C and the vacuum degree is 0.07Mpa. The time was 2 hours, and the distillation under reduced pressure was continued for 2 hours after the dropwise addition was completed. The emulsion is spray-dried, the inlet air temperature is 180°C, and the outlet air temperature is 80°C.
[0026] The appearance of the obtained powder oil is light yellow, good fluidity, a...
Embodiment 2
[0028] Starch Sodium Octenyl Succinate 90%
[0029] Mabofloxacin 10%
[0030] Preparation:
[0031] Dissolve starch sodium octenyl succinate in purified water to prepare a wall material solution with a concentration of 30%. Dissolve Mabofloxacin in a mixed solution of dichloromethane / absolute ethanol (3:2) to prepare a core material solution with a concentration of 50%. At a shear rate of 10000r / min, slowly drop the core material solution into the wall material solution, and carry out vacuum distillation at the same time to recover the dichloromethane / absolute ethanol mixed solution. The system temperature is 40°C and the vacuum degree is 0.01Mpa. The time was 0.5 hour, and the vacuum distillation was continued for 2 hours after the dropwise addition was completed. The emulsion is spray-dried, the inlet air temperature is 220°C, and the outlet air temperature is 120°C.
[0032] The appearance of the obtained powder oil is light yellow, good fluidity, and dissolves in water...
Embodiment 3
[0034] Starch Sodium Octenylsuccinate 70%
[0035] Mabofloxacin 30%
[0036] Preparation:
[0037] Dissolve starch sodium octenyl succinate in purified water to prepare a wall material solution with a concentration of 20%. Dissolve Mabofloxacin in a mixed solution of dichloromethane / absolute ethanol (19:3) to prepare a core material solution with a concentration of 35%. At a shear rate of 7500r / min, slowly drop the core material solution into the wall material solution, and at the same time carry out vacuum distillation to recover the mixed solution of dichloromethane / absolute ethanol. The system temperature is 25°C and the vacuum degree is 0.04Mpa. The time was 1.25 hours, and the distillation under reduced pressure was continued for 2 hours after the dropwise addition was completed. The emulsion is spray-dried, the inlet air temperature is 200°C, and the outlet air temperature is 100°C.
[0038] The appearance of the obtained powder oil is light yellow, good fluidity, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com