Application of cassia ketoside in preparation of xanthine oxidase inhibitor
A technology of xanthine oxidase and cassia ketone glycosides is applied in the application field of cassia ketone glycosides in the preparation of xanthine oxidase inhibitors, and can solve the problem that no ketoside glycosides inhibit the activity of xanthine oxidase and resist hyperuric acid Research reports on blood effects, etc., to achieve the effect of clear mechanism of action and less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The half inhibitory concentration IC 50 determination
[0020] HPLC determination method: Diamonsil ODS chromatographic column (250 mm × 4.6 mm, 5 mu m); mobile phase: 100 mmol / L sodium dihydrogen phosphate solution (pH=3.5); detection wavelength: 290 nm; flow rate: 1.0 mL / min.
[0021] Determination system: different concentrations of test product solutions (0.07 mol / L phosphate buffer containing 0.25% DMSO) 200 mu L added 0.07 mol / L phosphate buffer 140 mu L, xanthine oxidase solution 120 mu L (0.02 unit / mL, 0.07 mol / L phosphate buffer), keep warm at 25°C for 15 min, add xanthine solution 240 mu L (300 mu mol / L, 0.07 mol / L phosphate buffer), incubate at 25°C for 15 min, add 1 mol / L hydrochloric acid 100 mu L terminates the reaction. For blank control determination, the test solution was replaced with 0.07 mol / L phosphate buffer containing 1% DMSO. The amount of uric acid produced was determined by HPLC, and the concentration of each group was paralleled...
Embodiment 2
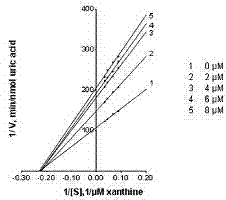
[0023] Determination of the inhibitory effect of cassiazone glycosides on xanthine oxidase
[0024] HPLC determination method: Diamonsil ODS chromatographic column (250 mm × 4.6 mm, 5 mu m); mobile phase: 100 mmol / L sodium dihydrogen phosphate solution (pH=3.5); detection wavelength: 290 nm; flow rate: 1.0 mL / min.
[0025] Determination system: different concentrations of test product solutions (0.07 mol / L phosphate buffer containing 0.25% DMSO) 200 mu L, add 0.07 mol / L phosphate buffer 140 mu L, add xanthine solution 240 mu L (100 mu mol / L, 50 mu mol / L, 37.5 mu mol / L, 25 mu mol / L, 0.07 mol / L phosphate buffer), xanthine oxidase solution 120 mu L (0.05 unit / mL, 0.07 mol / L phosphate buffer), keep warm at 25°C for 1 min, add 1 mol / L hydrochloric acid 100 mu L terminates the reaction. The amount of uric acid produced was determined by HPLC, and the concentration of each group was parallelized three times. The type of inhibition was determined by the Lineweaver-B...
Embodiment 3
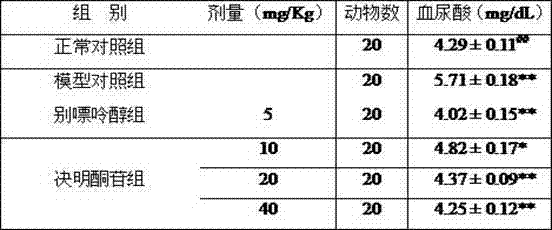
[0027] Effects of cassiatone glycosides on serum uric acid levels in experimental hyperuricemia model mice
[0028] The mice were randomly divided into 6 groups, 20 in each group, which were normal control group (normal saline 10 mL / Kg), model control group (oxonate potassium 250 mg / Kg), high-dose cassiazone glycoside group (oxygen Potassium oxonate 250 mg / Kg+cassarone 40 mg / Kg), middle-dose group (potassium oxonate 250 mg / Kg+cassarone 20 mg / Kg), low-dose group (Potassium oxonate 250 mg / Kg+cassine glycoside 10 mg / Kg), allopurinol control group (potassium oxonate 250 mg / Kg+allopurine 5 mg / Kg). Every day at 9:00 AM, the model control group and each administration group were administered intragastrically with oxonate potassium, and 1 hour later, each administration group was administered by intragastric administration, and the administration cycle was 7 days. The normal control group and the model control group were administered intragastrically equal volume of saline. After 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com