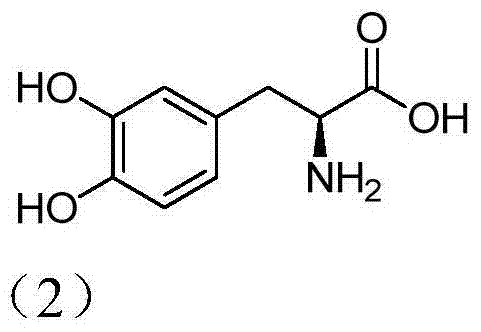
Preparation method for levodopa intermediate derivative
A technology of levodopa intermediates and derivatives is applied in the field of preparation of levodopa intermediate derivatives, and can solve the problems of complicated steps, high cost, unsuitable for industrial production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
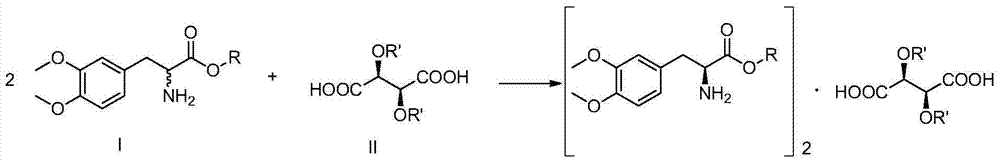
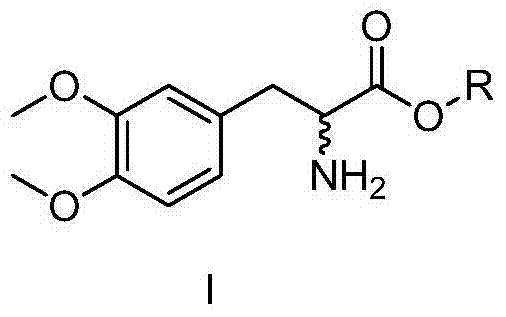
[0052] Resolution method of (±)-3,4-dimethoxyphenylalanine ethyl ester
[0053] Dissolve 4.96g (±)-3,4-dimethoxyphenylalanine ethyl ester in 30mL ethanol, and add a mixture consisting of 3.56g (+)-dibenzoyltartaric acid and 20mL ethyl acetate under stirring Solution, after standing at room temperature for 24 hours, a large amount of solids precipitated, filtered, washed with a little 3:2 mixed solvent of ethanol and ethyl acetate, and dried to obtain 3.24g [(-)-3,4-dimethoxybenzene ethyl alanine] 2 • (+)-Dibenzoyltartaric acid. The resolution chemical yield was 38.0%, and the optical content by HPLC was 97.2%.
[0054] The salt was placed in an aqueous solution of sodium carbonate (the amount of sodium carbonate used was 0.5 e.q.[(-)-3,4-dimethoxyphenylalanine ethyl ester] 2 ·(+)-dibenzoyl tartaric acid), the temperature is controlled below 5°C, reacted, extracted with ethyl acetate, and evaporated to get (-)-3,4-dimethoxyphenylalanine ethyl acetate.
Embodiment 2
[0056] Resolution method of (±)-3,4-dimethoxyphenylalanine ethyl ester
[0057] Dissolve 4.96g (±)-3,4-dimethoxyphenylalanine ethyl ester in 25mL ethanol, add 3.76g (+)-dibenzoyl tartaric acid monohydrate and 25mL ethyl acetate under stirring After standing at room temperature for 24 hours, a large amount of solids were precipitated, filtered, washed with a little 1:1 mixed solvent of ethanol and ethyl acetate, and dried to obtain 3.1g [(-)-3,4-dimethyl Oxyphenylalanine ethyl ester] 2 • (+)-Dibenzoyltartaric acid. The resolution chemical yield was 36.4%, and the optical content by HPLC was 97.7%.
[0058] The salt was placed in an aqueous solution of sodium carbonate (the amount of sodium carbonate used was 0.5 e.q.[(-)-3,4-dimethoxyphenylalanine ethyl ester] 2 ·(+)-dibenzoyl tartaric acid), the temperature is controlled below 5°C, reacted, extracted with ethyl acetate, and evaporated to get (-)-3,4-dimethoxyphenylalanine ethyl acetate.
Embodiment 3
[0060] Resolution method of (±)-3,4-dimethoxyphenylalanine methyl ester
[0061] Dissolve 4.96g of (±)-3,4-dimethoxyphenylalanine methyl ester in 30mL of methanol, and add a mixture consisting of 3.56g of (+)-dibenzoyltartaric acid and 15mL of ethyl acetate under stirring Solution, after standing at room temperature for 24 hours, a large amount of solids were precipitated, filtered, washed with a little 2:1 mixed solvent of methanol and ethyl acetate, and dried to obtain 3.38g [(-)-3,4-dimethoxybenzene Alanine methyl ester] 2 • (+)-Dibenzoyltartaric acid. The resolution chemical yield was 39.7%, and the optical content by HPLC was 97.4%.
[0062] The salt was placed in an aqueous solution of sodium carbonate (the amount of sodium carbonate used was 0.5 e.q.[(-)-3,4-dimethoxyphenylalanine methyl ester] 2 ·(+)-dibenzoyl tartaric acid), the temperature is controlled below 5°C, reacted, extracted with ethyl acetate, and evaporated to get (-)-3,4-dimethoxyphenylalanine acid met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com