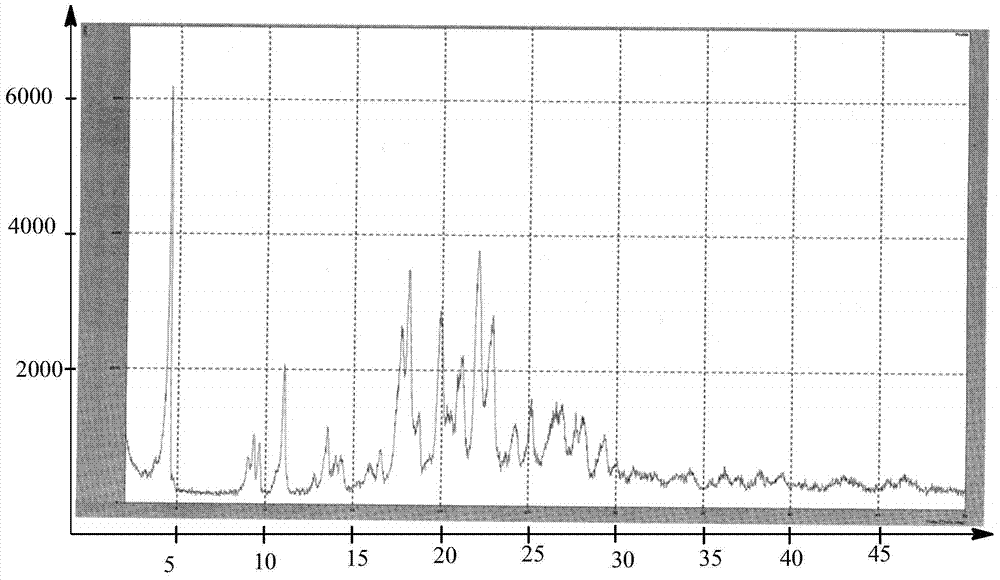
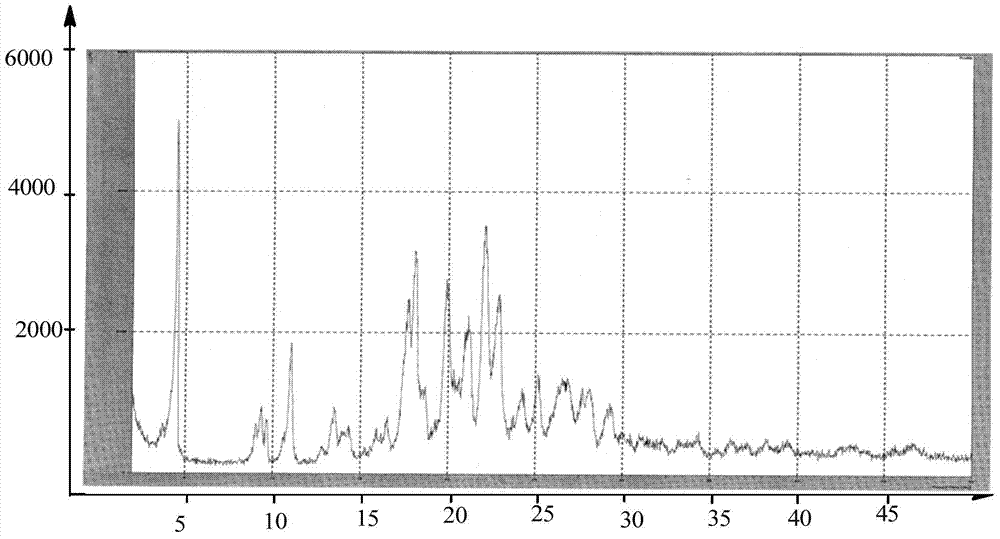
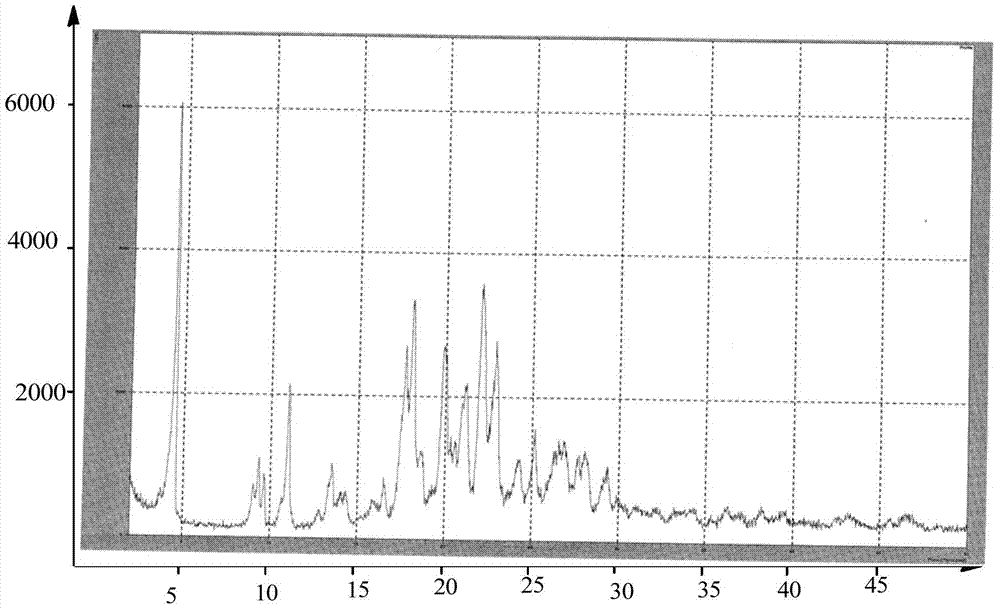
Preparing method of dabigatran etexilate mesylate crystal form I
A technology of dabigatran etexilate mesylate and dabigatran etexilate mesylate, applied in the field of preparation of dabigatran etexilate mesylate I crystal form, can solve the problem of unstable preparation of dabigatran etexilate mesylate Ⅰ crystal form and other issues, to achieve the effect of suitable process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) at room temperature, dabigatran etexilate is joined in acetone, wherein, acetone volume consumption is 20 times of dabigatran etexilate quality; be warming up to 30 ℃ and make dabigatran etexilate fully dissolve; then cool down to 25 ℃, keep temperature 25 ℃, add methanesulfonic acid acetone solution, wherein, the amount of methanesulfonic acid substance is 0.98 times of the amount of dabigatran etexilate substance, and the volume consumption of acetone is 15 times of methanesulfonic acid quality; Mix well , making dabigatran etexilate mesylate solution;
[0045] (2) Cool the mixture obtained in step (1) to 10°C, stir and crystallize for 1.5 hours;
[0046] (3) filter step (2) gained mixture, filter cake is joined in the ethyl acetate solvent, wherein, the ethyl acetate solvent volume consumption is 30 times of dabigatran etexilate quality described in the step (1); Warming up to 45°C, keep the temperature at 45°C, and stir for 5 hours;
[0047] (4) cooling the mi...
Embodiment 2
[0052] (1) at room temperature, dabigatran etexilate is joined in acetone, wherein, acetone volume consumption is 20 times of dabigatran etexilate quality; be warming up to 56 ℃ and make dabigatran etexilate dissolve completely; then cool down to 40 ℃, keep temperature 40 ℃, add methanesulfonic acid acetone solution, wherein, the amount of methanesulfonic acid substance is 0.98 times of the amount of dabigatran etexilate substance, and the volume consumption of acetone is 15 times of methanesulfonic acid quality; Mix well , making dabigatran etexilate mesylate solution;
[0053] (2) Cool the mixture obtained in step (1) to 15°C, stir and crystallize for 1.5 hours;
[0054] (3) filter step (2) gained mixture, filter cake is joined in the ethyl acetate solvent, wherein, the ethyl acetate solvent volume consumption is 15 times of dabigatran etexilate quality described in the step (1); Warming up to 55°C, keep the temperature at 55°C, and stir for 1 hour;
[0055] (4) cooling th...
Embodiment 3
[0061] (1) at room temperature, dabigatran etexilate is joined in acetone, wherein, acetone volume consumption is 20 times of dabigatran etexilate quality; be warming up to 40 ℃ and make dabigatran etexilate dissolve completely; then cool down to 30 ℃, keep temperature 30 ℃, add methanesulfonic acid acetone solution, wherein, the amount of methanesulfonic acid substance is 0.98 times of the amount of dabigatran etexilate substance, and the volume consumption of acetone is 15 times of methanesulfonic acid quality; Mix well , making dabigatran etexilate mesylate solution;
[0062] (2) Cool the mixture obtained in step (1) to 12°C, stir and crystallize for 1.5 hours;
[0063] (3) filter step (2) gained mixture, filter cake is joined in the methyl propionate solvent, wherein, the methyl propionate solvent volume consumption is 5 times of dabigatran etexilate solid mass described in step (1) ;Heat up to 78°C, keep the temperature at 78°C, and stir for 2 hours;
[0064] (4) Cool t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com