Monoclonal antibody specifically combined with HFABP (heart fatty acid binding protein) and applications thereof
A monoclonal antibody and protein technology, applied in anti-animal/human immunoglobulin, microbial-based methods, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
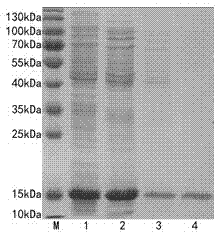
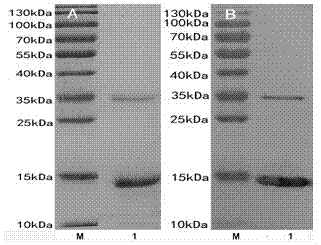
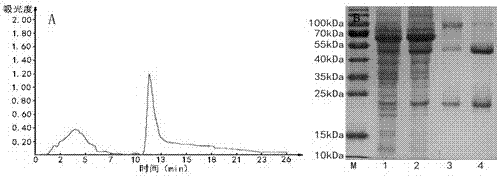
[0042] 1. Expression and Purification of Recombinant Strain HFABP Protein
[0043] The recombinant prokaryotic expression plasmid of HFABP was transformed into Escherichia coli ER2566, and spread to the + After culturing at 37 °C for 12 h, a single colony was picked from the plate and inoculated into 10 mL LB medium (containing Kan + ), cultured at 37 ℃ for 8 h, and then inoculated 10 mL of cultured bacteria into 1L LB medium (containing Kan + ) cultured in the bacterial solution OD 600 When the value reached 0.6, the expression was induced by adding IPTG at a final concentration of 0.4 mmol / L. After 4 hours, collect the cells by centrifugation at 12,000 g at room temperature for 5 minutes, add 40 mL of cell lysate (50 mmol / L Tris HCl, 1 mmol / L EDTA, 100 mmol / L NaCl, pH 8.0), Ф10 mm probe with 200W power The cells were sonicated, centrifuged at 12,000 g for 10 min at 4°C, and the supernatant was collected. The supernatant was precipitated with ammonium sulfate, dialyzed...
Embodiment 2
[0049] Freund's complete adjuvant and Freund's incomplete adjuvant containing heat-inactivated Mycobacterium tuberculosis were shaken and mixed thoroughly on a vortex shaker to ensure that the bacterial solution was fully suspended. Phosphate buffer and 1.0 mg / mL recombinant HFABP were mixed at 1:1 (V / V), and then the mixture was pumped repeatedly with a 1.0 mL syringe until the recombinant protein and adjuvant were fully mixed to form a homogeneous emulsion.
[0050] Inject 6-8 w SPF female BALB / c mice with complete Freund's adjuvant and heat-inactivated Mycobacterium tuberculosis emulsified immunization antigen HFABP to each mouse with 40 μg of antigen from the hock joint, the first immunization Two weeks later, each mouse was re-injected with 40 μg antigen from the hock joint with incomplete Freund's adjuvant and heat-inactivated Mycobacterium tuberculosis emulsified immunization antigen HFABP. Eight days after immunization, blood was collected from the tail vein of the mic...
Embodiment 3
[0055] 1. Select sp2 / 0 myeloma cells in good growth state and lymphocytes from immunized mice to mix at a ratio of about 1:2-1:3, centrifuge at 1200 g for 2 min, and discard the supernatant. Tap the centrifuge tube to disperse the cells evenly, slowly add 1 mL of 37 ℃ preheated PEG 1500, mix gently, and immediately add 20 mL of serum-free 1640 medium after 60 s. After centrifugation at 1000 g for 2 min, discard the supernatant, add 20% FBS serum HAT-1640 medium to the cell pellet and mix gently, evenly spread in 96-well plate, 200 μL per well, at 37 °C in 5% CO 2 cultured in an incubator. After 5-7 days, observe the fusion effect and change the medium. After 3 days, take the supernatant and use the indirect ELISA method to screen positive clones. Select wells with a higher ratio of positive value to cell number, and perform subcloning by limiting dilution method. After 4 times of subcloning, a hybridoma cell line capable of stably secreting antibodies is obtained.
[0056]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com