Production process of aqueous sodium hypochlorite solution
A technology of sodium hypochlorite and its manufacturing method, which is applied in the direction of hypochlorous acid, hypochlorite, chemical instruments and methods, and can solve problems such as increased decomposition and deterioration of consumed resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
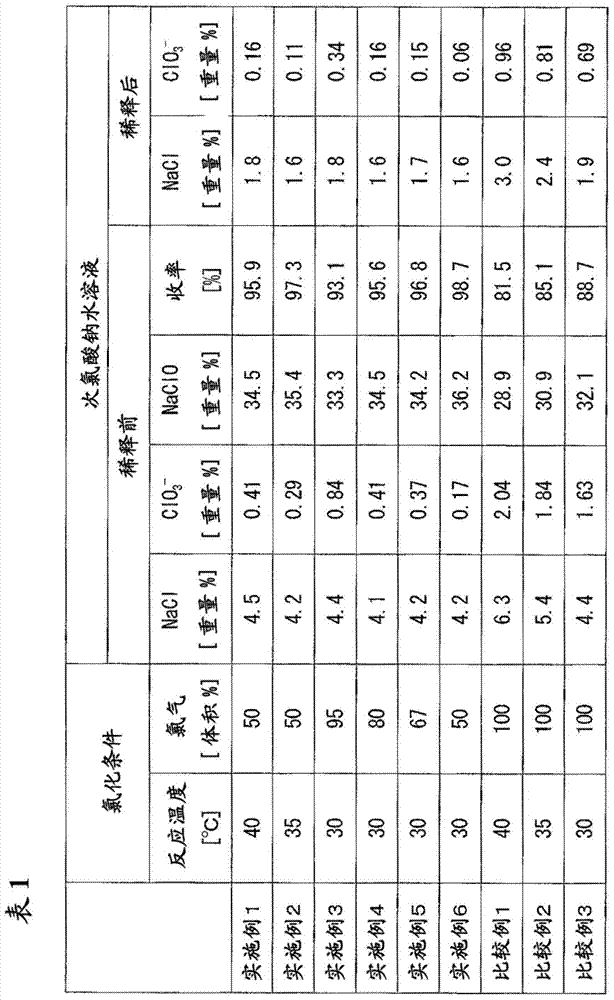
[0061] To a reaction tank equipped with a stirrer, a coil cooler, and an external circulation cooler, a 45% by mass aqueous sodium hydroxide solution was supplied as a raw material at 1514 kg / hour while stirring, and the aqueous sodium hydroxide solution was maintained at 40° C. , and 560 kg / hour of chlorine gas was diluted to 50% by volume with nitrogen gas and introduced, and the chlorination reaction was carried out so that the residence time became about 100 minutes.
[0062] Extract the reactant slurry at 2074kg / hour from the reaction tank, and carry out solid-liquid separation with a centrifuge, thus obtaining 682kg / hour of precipitated sodium chloride and 1390kg / hour of sodium hypochlorite concentration of 34.5% by mass, sodium chloride concentration It is 4.5 mass %, and the low-salt sodium hypochlorite aqueous solution whose chlorate ion concentration is 0.41 mass %. The yield at this time was 95.9%. It should be noted that the yield is a value calculated from the nu...
Embodiment 2
[0065] To a reaction tank equipped with a stirrer, a coil cooler, and an external circulation cooler, a 45% by mass aqueous sodium hydroxide solution was supplied as a raw material at 1512 kg / hour while stirring, and the aqueous sodium hydroxide solution was maintained at 35° C. , and 560 kg / hour of chlorine gas was diluted to 50% by volume with nitrogen gas and introduced, and the chlorination reaction was carried out so that the residence time became about 100 minutes.
[0066] Extract the reactant slurry at 2072kg / hour from the reaction tank, and carry out solid-liquid separation with a centrifuge, thus obtaining 680kg / hour of precipitated sodium chloride and 1390kg / hour of sodium hypochlorite concentration of 35.4% by mass, sodium chloride concentration It is 4.2 mass %, and the low-salt sodium hypochlorite aqueous solution whose chlorate ion concentration is 0.29 mass %. The yield at this time was 97.3%.
[0067] The obtained low-salt sodium hypochlorite aqueous solution...
Embodiment 3
[0069] To a reaction tank equipped with a stirrer, a coil cooler, and an external circulation cooler, a 45% by mass aqueous sodium hydroxide solution was supplied as a raw material at 1513 kg / hour while stirring, and the aqueous sodium hydroxide solution was maintained at 30° C. , and 556 kg / hour of chlorine gas was diluted to 95% by volume with nitrogen gas and introduced, and the chlorination reaction was carried out so that the residence time became about 100 minutes.
[0070] Extract the reactant slurry with 2069kg / hour from the reaction tank, and carry out solid-liquid separation with a centrifuge, thus obtaining 642kg / hour of sodium chloride and 1427kg / hour of sodium hypochlorite concentration of 33.3% by mass, sodium chloride concentration It is 4.4 mass %, and the low-salt sodium hypochlorite aqueous solution whose chlorate ion concentration is 0.84 mass %. The yield at this time was 93.1%.
[0071] The obtained low-salt sodium hypochlorite aqueous solution was dilute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com