Composition for inhibiting melanin synthesis
A technology for inhibiting melanin and composition, which is applied in skin care preparations, cosmetics, etc., can solve the problems of limited inhibition effect, and achieve strong inhibition ability and less irritating effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Culture and treatment of mouse B16F10 and Melan-a melanocytes
[0030] B16 F10 cells were purchased from the American Type Culture Collection (ATCC, Rock-vile, MD). Cells were cultured in DMEM (GIBCO, GRAND ISLAND, NY) medium containing 10% fetal bovine serum (GIBCO, GRAND ISLAND, NY) and 1% double antibody (penicillin 100U / mL, streptomycin 100ug / mL), placed At 37°C, 5% CO 2cultured under normal conditions. Melan-a cells were donated by the Chinese University of Hong Kong. Cells were cultured in RPMI1640 (GIBCO, GRAND ISLAND, NY) medium containing 10% fetal bovine serum (GIBCO, GRAND ISLAND, NY) and 1% double antibody (penicillin 100U / mL, streptomycin 100ug / mL), placed At 37°C, 5% CO 2 cultured under normal conditions. Trans dihydromorin was dissolved in 100% DMSO solution for storage, and diluted to the corresponding concentration with cell culture medium before use. The DMSO concentration in the final medium does not exceed 0.1%.
Embodiment 2
[0032] CCK-8 method cell viability test
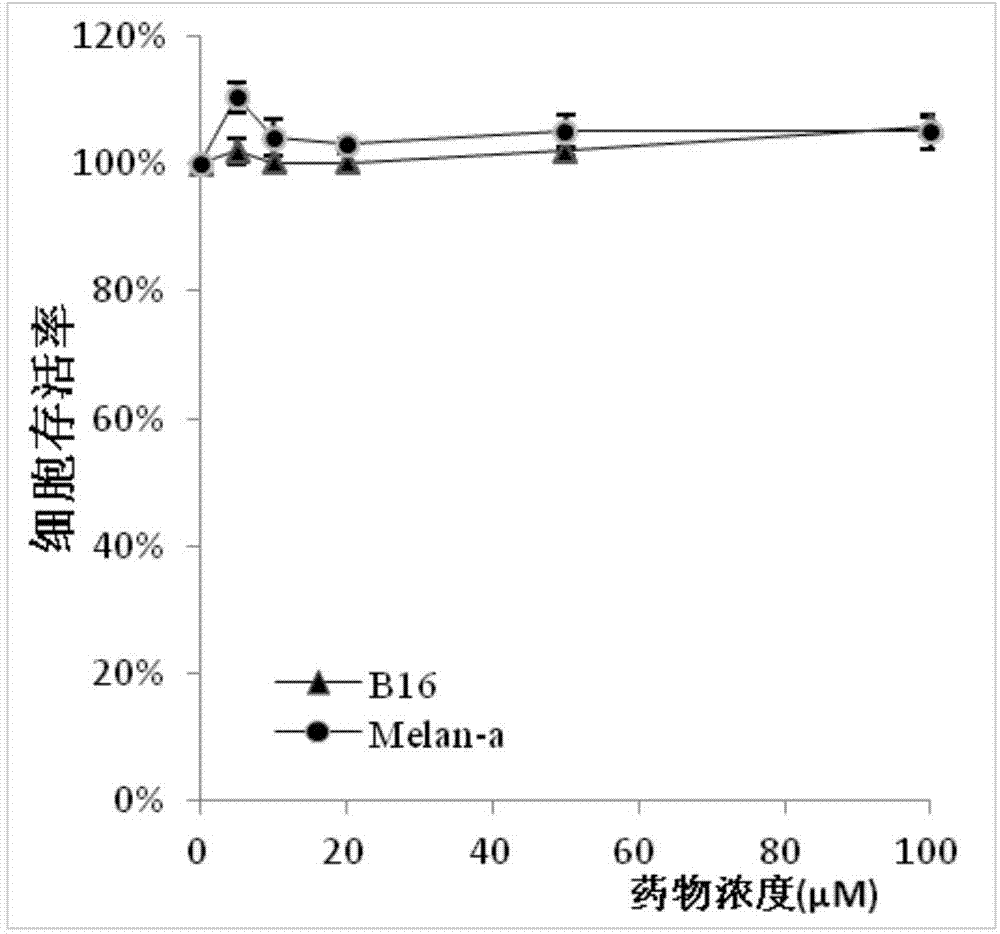
[0033] CCK-8 method cell viability test is used to determine the safe experimental concentration of the extract. 100 μL of B16 F10 cells (1x104 / well) or Melan-a cells (2x104 / well) were inoculated into 96-well plates 24 hours before the experiment, and treated with different concentrations of trans-morin and kojic acid for 72 hours, The cells were washed with PBS to remove the medium and the added compounds, and 10 μL of CCK-8 solution was added to each well for 2 hours, and the absorbance value was read at a wavelength of 450 nm by an enzyme-linked immunosorbent assay (Bio-Rad). Cell survival rate = (OD value of experimental group - OD value of blank group) / (OD value of control group - OD value of blank group). Each result is represented by the mean±S.D of the results of three independent experiments.
[0034] Such as figure 1 As shown, when the concentration is lower than 100 μM, in two different melanocytes, trans-dihydromorinin...
Embodiment 3
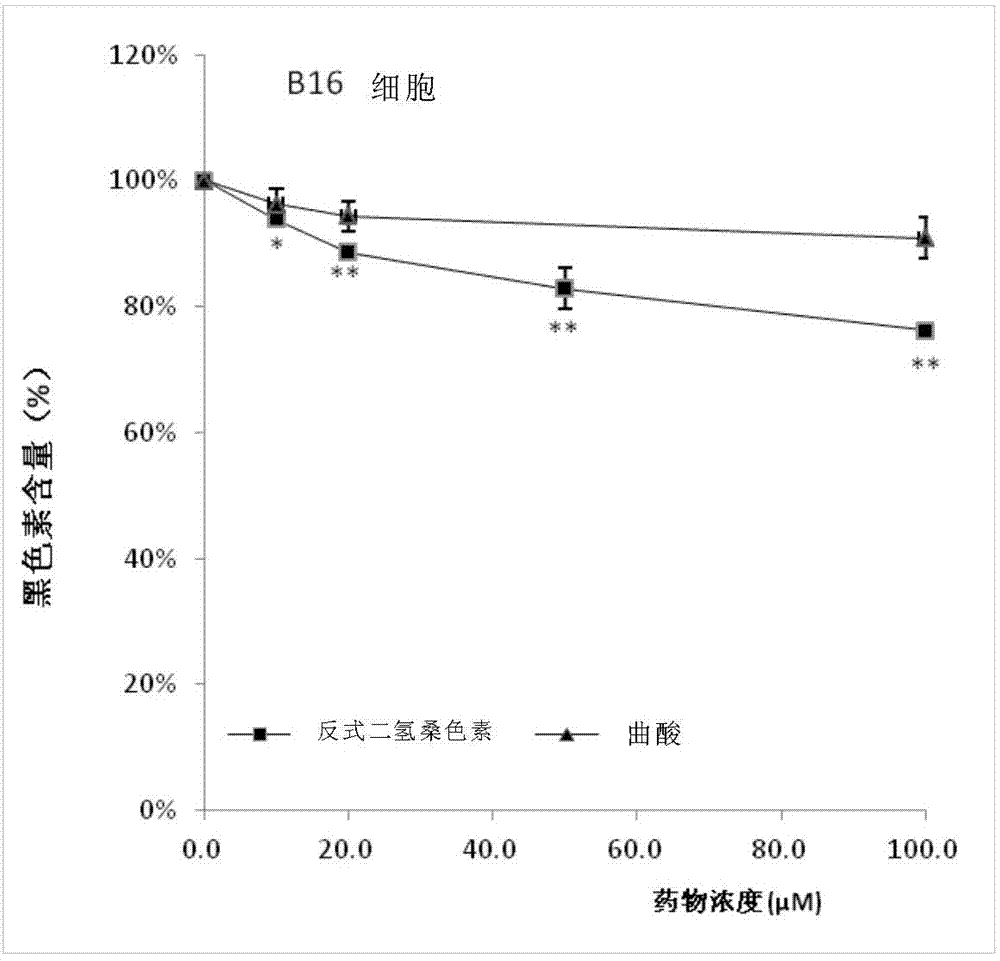
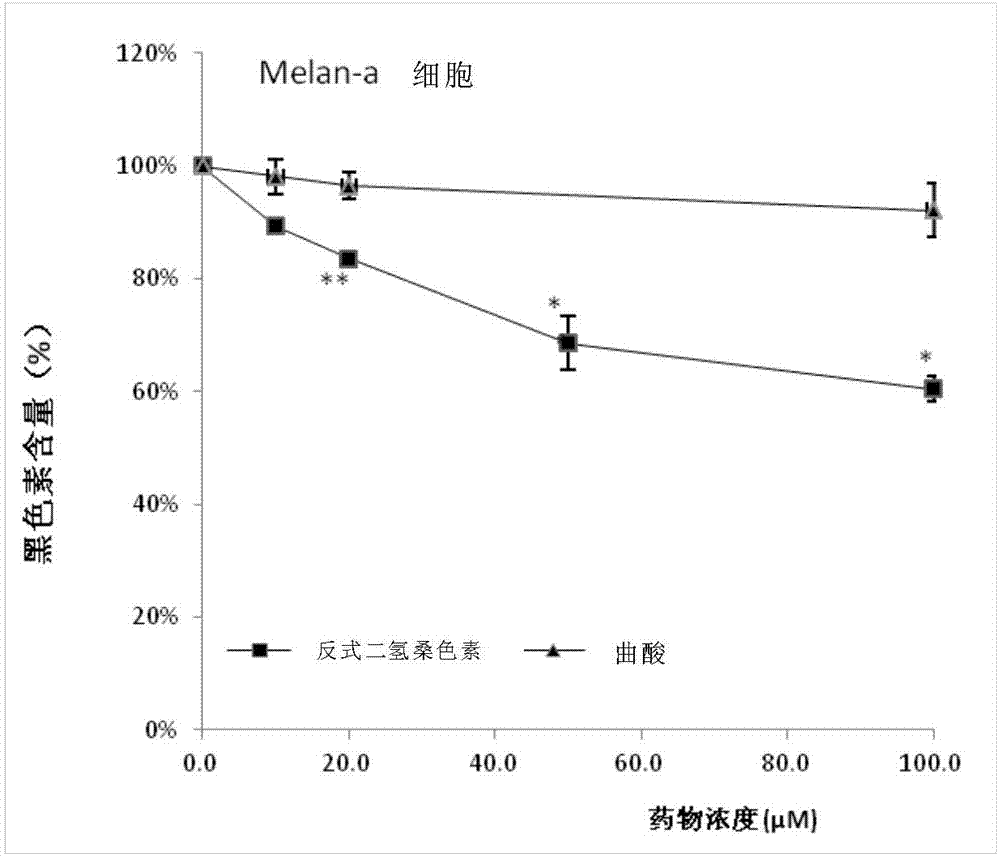
[0036] SRB method for cell number statistics and cell melanin content test
[0037] The SRB method was used to count the number of corresponding cells before the test of melanin content, so as to ensure that the reduction of melanin content was not due to the massive death of cells. Inoculate B16F10 or Melan-a cells (3x105 cells / well) in a 12-well plate, culture for 24 hours, and use trans-dihydromorin and α-MSH (1μM), and without trans-dihydromorin α-MSH (1 μM) was replaced once with fresh medium, and the incubation was continued for 72 hours. After the end of the culture, first use the SRB method to count the number of cells in each well: add 250 μL of trichloroacetic acid (4°C) to the cell culture medium in each well to fix the cells on the wall of the 12-well plate, and after fixing for 1 hour, wash the cells with PBS for 5 times to remove trichloroacetic acid, medium, and dead cells. After the 12-well plate was air-dried, 500 μL of 1% acetic acid solution containing 0.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com