Double-functional nano-composite spheres based on metal ion-inducing polypeptide self-assembly and preparation method thereof
A nano-composite, metal ion technology, applied in the direction of drug combinations, preparations for in vivo tests, non-active ingredients of polymer compounds, etc., can solve the problems of difficult diagnosis and detection of cancer, delay the best treatment period, etc., and achieve synthesis cost Inexpensive, simple steps, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Dissolve 0.5 mg of the polypeptide solid obtained by hydrolyzing α-lactalbumin under the action of serine protease in 1 mL of tris solution with a concentration of 75 mM and a pH of 7.5, and shake to dissolve completely; The pH value of aminomethane solution is adjusted with hydrochloric acid;
[0025] (2) Add 120 μL of Bengal Red solution with a concentration of 1 mM to the solution in step (1), mix and shake well;
[0026] (3) 100 μL of GdCl with a concentration of 10 mM 3 solution, and 40 μL of 10 mM CaCl 2 The solution is added to the solution in step (2), and then incubated with shaking at 37°C for 24 hours;
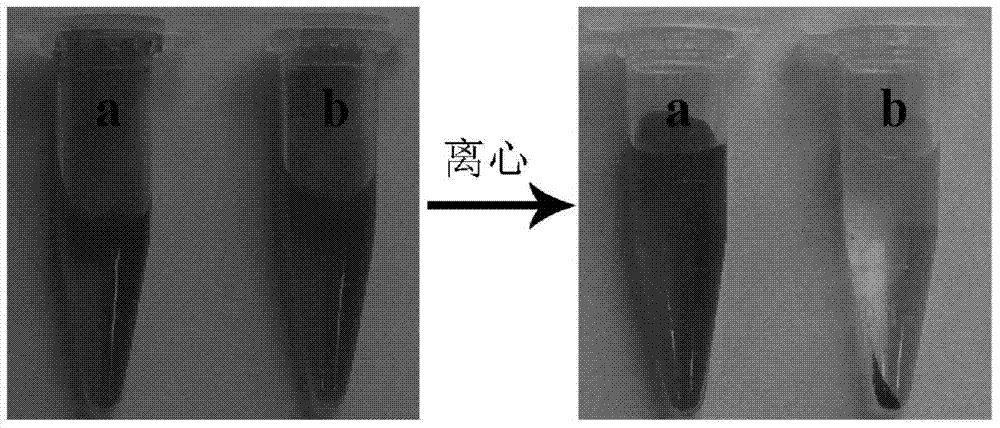
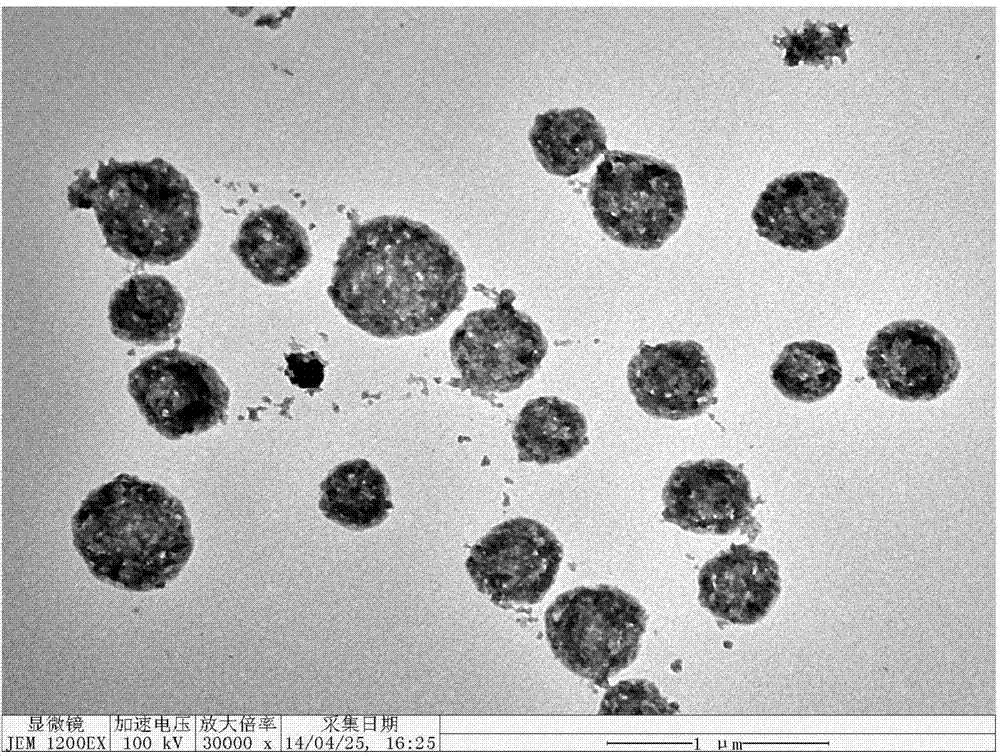
[0027] (4) After the reaction, the solid was collected by centrifugation, which was a bifunctional nanocomposite sphere based on metal ion-induced polypeptide self-assembly, and finally dispersed in 0.5 mL of water.
[0028] Add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride EDC of 1.05mg and the N-hydroxysuccinimide of 0.15mg in the dispe...
Embodiment 2
[0030] (1) Dissolve 0.5 mg of a polypeptide solid obtained by hydrolyzing α-lactalbumin under the action of serine protease in 1 mL of a tris solution with a concentration of 75 mM and a pH of 6.5, and shake to dissolve completely; The pH value of aminomethane solution is adjusted with hydrochloric acid;
[0031] (2) Add 120 μL of Bengal Red solution with a concentration of 1 mM to the solution in step (1), mix and shake well; (3) add 40 μL of GdCl with a concentration of 10 mM 3 solution, and 40 μL of 10 mM CaCl 2 The solution is added to the solution in step (2), and then incubated with shaking at 37°C for 24 hours;
[0032] (4) After the reaction, the solid was collected by centrifugation, which was a bifunctional nanocomposite sphere based on metal ion-induced polypeptide self-assembly, and finally dispersed in 2 mL of water.
[0033] Add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride EDC of 1.05mg and the N-hydroxysuccinimide of 0.15mg in the dispersion liq...
Embodiment 3
[0035](1) Dissolve 0.5 mg of the polypeptide solid obtained by hydrolyzing α-lactalbumin under the action of serine protease in 1 mL of tris solution with a concentration of 75 mM and a pH of 7.5, and shake to dissolve completely; The pH value of aminomethane solution is adjusted with hydrochloric acid;
[0036] (2) Add 120 μL of Bengal Red solution with a concentration of 1 mM to the solution in step (1), mix and shake well;
[0037] (3) 40 μL of GdCl with a concentration of 10 mM 3 solution, and 50 μL of 10 mM CaCl 2 The solution is added to the solution in step (2), and then incubated with shaking at 37°C for 24 hours;
[0038] (4) After the reaction, the solid was collected by centrifugation, that is, the bifunctional nanocomposite sphere based on metal ion-induced polypeptide self-assembly, and finally dispersed in 1 mL of water.
[0039] Add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride EDC of 1.05mg and the N-hydroxysuccinimide of 0.15mg in the dispersi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Longitudinal relaxation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com