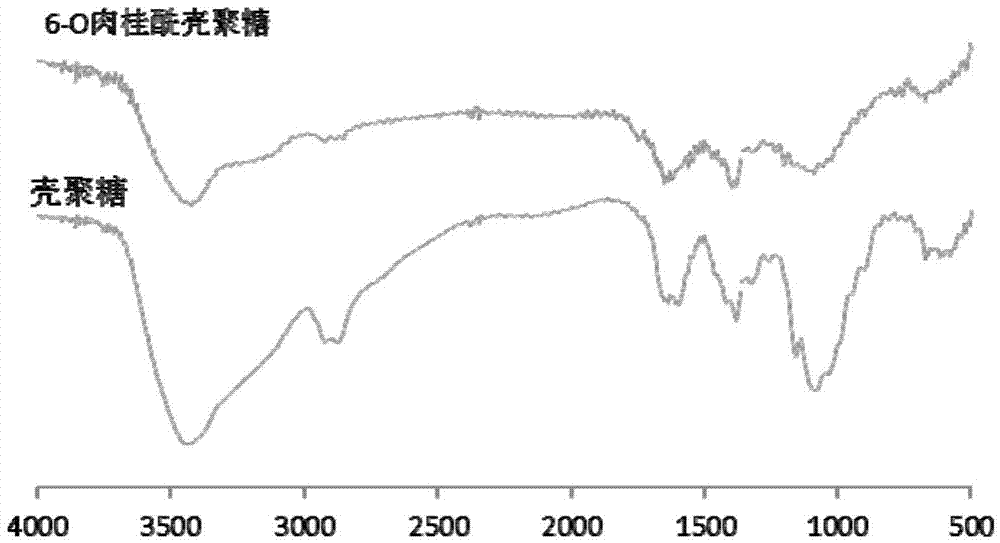
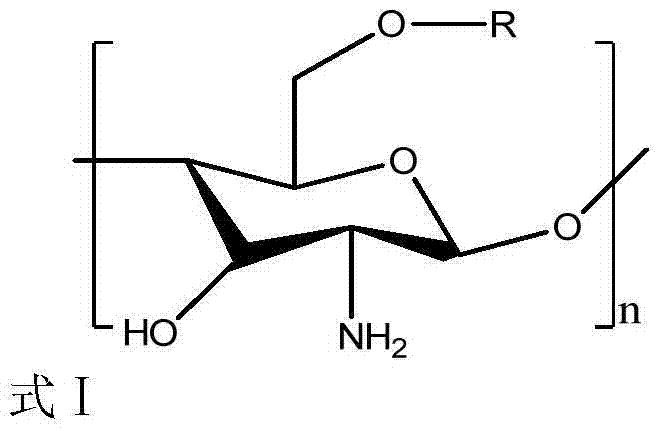
Novel chitosan grafted cinnamoyl product as well as preparation method and application thereof
A technology for grafting cinnamoyl and chitosan to chitosan is applied in the field of novel chitosan-grafting cinnamoyl products and the preparation thereof, and can solve the problems affecting the sustainable development of agriculture, human health, food safety and environmental pollution, etc. , to overcome the poor water solubility, expand the application field, and achieve the effect of good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 4.0g (27mmol) cinnamic acid was placed in a 200ml round bottom flask, dissolved in 110mL 1,2-dichloroethane, and then 5.4mL SOCl was added 2 , Refluxed in an oil bath at 50-60°C, tracked by thin layer chromatography (TLC), and the reaction was completed after 2 hours. Remove 1,2-dichloroethane and excess SOCl under reduced pressure 2 , recrystallized from petroleum ether, and evaporated the solvent under reduced pressure to obtain cinnamoyl chloride.
[0028] 3.0g (about 18mmol) chitosan (MW=325kDa), 60mL dimethylformamide (water containing 5% (v / v)), stirred at room temperature to make chitosan evenly swell and disperse in the solvent, and then Add 8g of phthalic anhydride (54mmol), stir and reflux at 120°C for 8h, then cool to room temperature, pour the reactant into ice water, filter under reduced pressure and wash with distilled water, after filtration, disperse the product in ethanol, wash completely with ethanol, Filter and dry to obtain brown powdery solid N-ph...
Embodiment 2
[0035] Solubility measurement: 0.5g of the product of Example 1 was suspended in 10ml of water, stirred at 25°C for 2h. By centrifugation at 5000rpm for 15min, the supernatant was removed. The precipitate was washed completely with ethanol. The precipitate was dried in a phosphorus pentoxide vacuum desiccator and weighed. By calculating the percentage of chitosan dissolved, the solubility of chitosan and cinnamoyl chitosan were 0.416g / 100mL and 0.805g / 100mL respectively.
Embodiment 3
[0037]The antibacterial activity of the tested samples was determined by the growth rate method. The test sample was prepared with sterilized water to a concentration of 2 mg / ml, and the chitosan was prepared with 0.07% (V / V) acetic acid solution. Measure 1ml of the sample solvent into a graduated test tube, pour the melted PDA medium into the volume to 10ml, and wait for the medium to cool and solidify to obtain a drug-mixed plate. Use a puncher (diameter 4mm) to punch the pre-cultivated test bacteria into a cake, place the test bacteria cake upside down on the center of the medium surface, do 3 repetitions for each treatment, and set the corresponding solvent control. After culturing at a constant temperature of 25°C for 72 hours, the colony expansion diameter was measured by the cross method, and the mycelial growth inhibition rate was calculated according to the following formula (see Table 1).
[0038]
[0039] Table 1 Antibacterial activity of chitosan grafted cinnam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com