Sodium nitrite determination method
A sodium nitrite and determination method technology, applied in the direction of color/spectral characteristic measurement, etc., can solve the problems of harm, strong carcinogenicity of reagents, easy formation of secondary pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
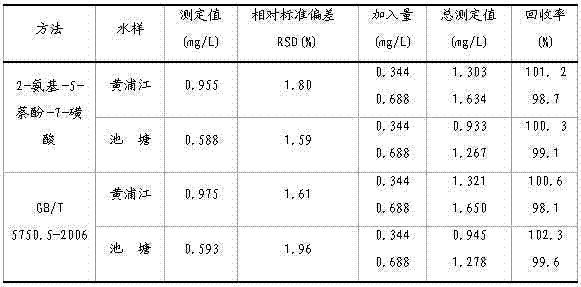
Embodiment 1
[0029] A kind of assay method of sodium nitrite, specifically comprises the following steps:
[0030](1) Preparation of blank reagents
[0031] ①. Use a pipette to pipette 10.0mL of deionized water into a 25mL colorimetric tube, and add 1.0mL of potassium bromide aqueous solution with a mass percentage concentration of 1%, 1.0×10 -2 1.0mL of mol / L p-aminobenzenesulfonic acid aqueous solution, then add 1.0mL of 1.5 mol / L hydrochloric acid aqueous solution, shake well to obtain deionized water diazonium salt solution, let the deionized water diazonium salt solution stand for 1min;
[0032] ② Take another 25mL volumetric flask, add 3mL of 1.0mol / L sodium carbonate aqueous solution and 1.0×10 -2 mol / L 2-N-ethyl-5-naphthol-7-sulfonic acid aqueous solution 1.0mL, transfer the deionized water diazonium salt solution in the colorimetric tube of step ① to the above-mentioned sodium carbonate aqueous solution and 2-N-ethyl-5-naphthol-7-sulfonic acid aqueous solution in the volumetric ...
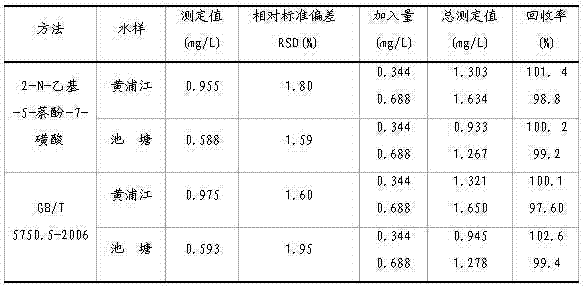
Embodiment 2
[0047] A kind of assay method of sodium nitrite, specifically comprises the following steps:
[0048] (1) Preparation of blank reagents
[0049] ①. Use a pipette to pipette 10.0mL of deionized water into a 25mL colorimetric tube, and add 1.0mL of potassium bromide aqueous solution with a mass percentage concentration of 1%, 1.0×10 -2 mol / L p-aminobenzenesulfonic acid aqueous solution 1.0mL, then add 1.5 mol / L hydrochloric acid aqueous solution 1.0mL, shake well to obtain deionized water diazonium salt solution, let the deionized water diazonium salt solution stand for 3min;
[0050] ② Take another 25mL volumetric flask, add 3mL of 1.0mol / L sodium carbonate aqueous solution and 1.0×10 -2 mol / L 2-N-ethyl-5-naphthol-7-sulfonic acid aqueous solution 1.0mL, transfer the deionized water diazonium salt solution in the colorimetric tube of step ① to the above-mentioned sodium carbonate aqueous solution and 2-N-ethyl-5-naphthol-7-sulfonic acid aqueous solution in the volumetric flask...
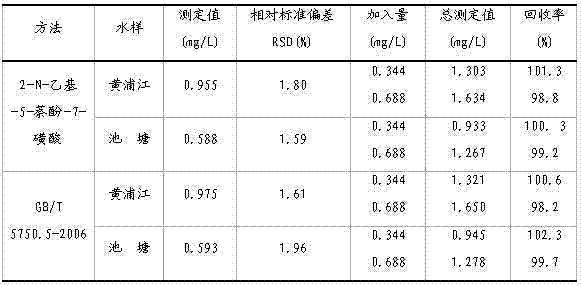
Embodiment 3
[0066] A kind of assay method of sodium nitrite, specifically comprises the following steps:
[0067] (1) Preparation of blank reagents
[0068] ①. Use a pipette to pipette 10.0mL of deionized water into a 25mL colorimetric tube, and add 1.0mL of potassium bromide aqueous solution with a mass percentage concentration of 1%, 1.0×10 -2 1.0mL of mol / L p-aminobenzenesulfonic acid aqueous solution, then add 1.0mL of 1.5 mol / L hydrochloric acid aqueous solution, shake well to obtain deionized water diazonium salt solution, let the deionized water diazonium salt solution stand for 5min;
[0069] ② Take another 25mL volumetric flask, add 3mL of 1.0mol / L sodium carbonate aqueous solution and 1.0×10 -2 mol / L 2-N-ethyl-5-naphthol-7-sulfonic acid aqueous solution 1.0mL, transfer the deionized water diazonium salt solution in the colorimetric tube of step ① to the above-mentioned sodium carbonate aqueous solution and 2-N-ethyl-5-naphthol-7-sulfonic acid aqueous solution in the volumetric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com