Hyaluronic acid-stabilized high relaxivity gadolinium oxide magnetic resonance nanoprobes
A technology of hyaluronic acid and nanoprobes, which can be applied in preparations for in vivo experiments and pharmaceutical formulations, can solve the problems that gadolinium-based contrast agents cannot meet special imaging requirements, reduce immune reactions in vivo, and make preparation conditions simple , reducing the effect of the immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1) In a beaker, dissolve 0.2g of enzymatically cleaved hyaluronic acid sodium salt with a molecular weight of 5000 in 10ml of water, and stir until completely dissolved. Add 1mL of 0.1M Gd(NO 3 ) 3 ·6H 2 O aqueous solution, in which hyaluronic acid sodium salt and Gd(NO 3 ) 3 ·6H 2 The mass ratio of O was 40:9. After 5 min, NaOH aqueous solution was added to adjust the pH to 10. After continuing to stir at room temperature for 1 h, a colorless liquid was obtained;
[0021] 2) Put the obtained hyaluronic acid gadolinium oxide nanomaterial into a dialysis bag (molecular weight cut-off is 8000-14000Da), and purify. The dialysis time is 24 hours, and the water is changed every 4 hours. After dialysis, a total volume of 50 mL of purified nanoprobe solution was obtained;
[0022] 3) Obtain a flocculent white solid product by freeze-drying, which is a gadolinium oxide magnetic resonance nanoprobe;
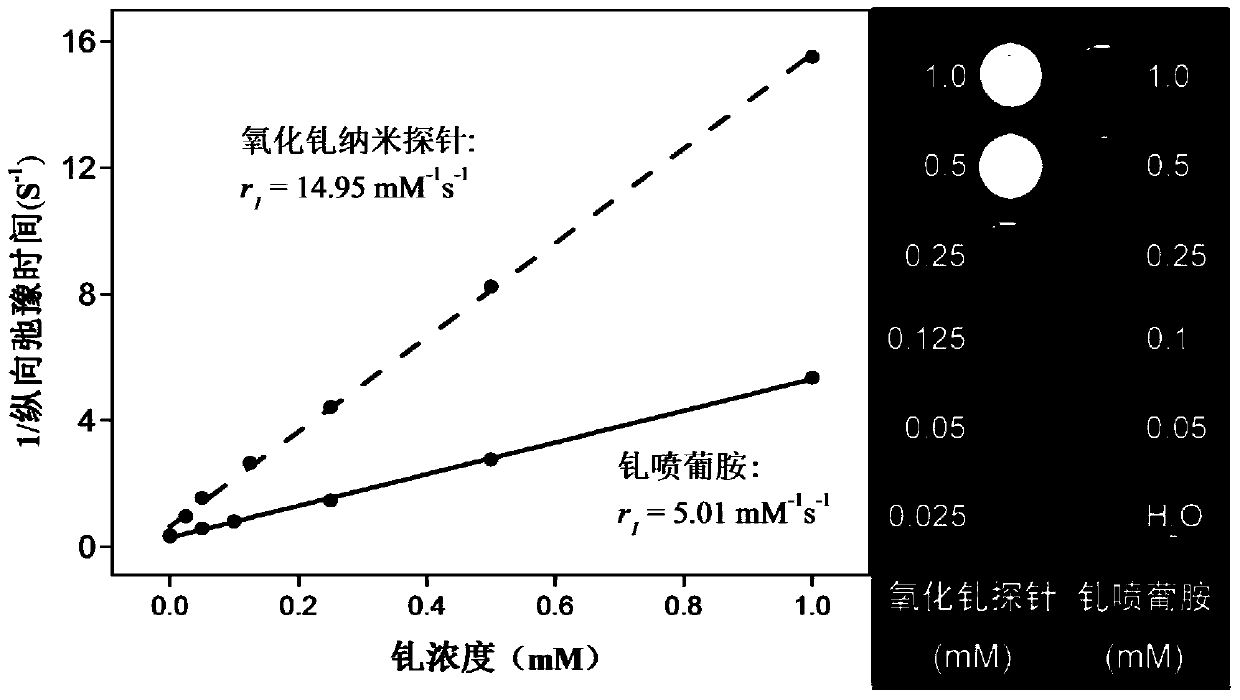
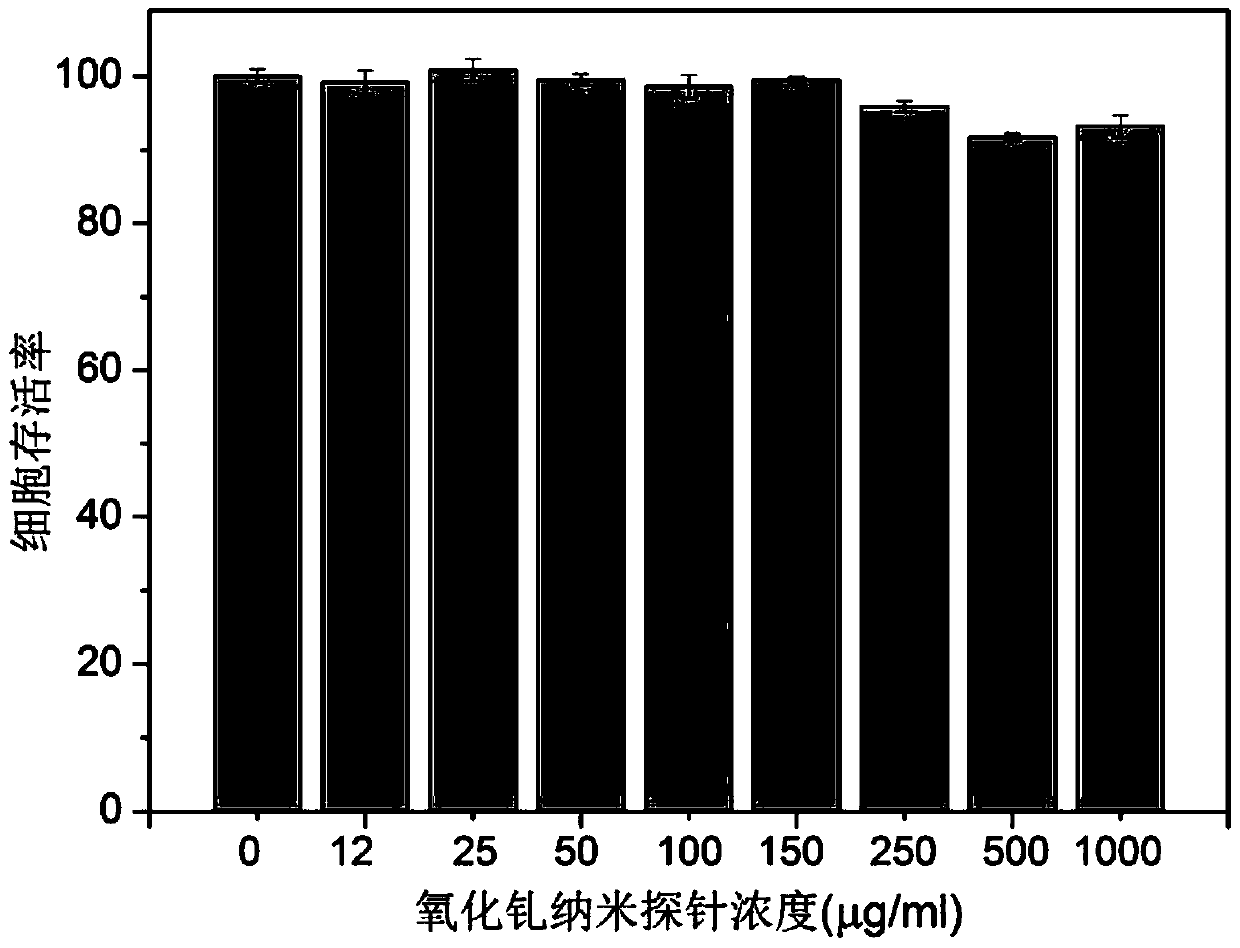
[0023] Get 20mg of the magnetic resonance imaging probe solid prepared...
Embodiment 2
[0025] A method for preparing a gadolinium oxide magnetic resonance imaging nanoprobe with good biocompatibility, the steps and methods are basically the same as in Example 1, except that the sodium hyaluronic acid and Gd(NO 3 ) 3 ·6H 2 O mass ratio is 200:9.
[0026] Get 20mg of the magnetic resonance imaging probe solid prepared by this embodiment and carry out high power transmission electron microscope characterization, T 1 Relaxation rate and T 1 Weighted imaging and in vitro cytotoxicity test MTT, the test results are similar to Example 1.
Embodiment 3
[0028] A method for preparing a gadolinium oxide magnetic resonance imaging nanoprobe with good biocompatibility, the steps and methods are basically the same as in Example 1, except that the sodium hyaluronic acid and Gd(NO 3 ) 3 ·6H 2 The O mass ratio is 80:9.
[0029]Get 20mg of the magnetic resonance imaging probe solid prepared by this embodiment and carry out high power transmission electron microscope characterization, T 1 Relaxation rate and T 1 Weighted imaging and in vitro cytotoxicity test MTT, the test results are similar to Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com