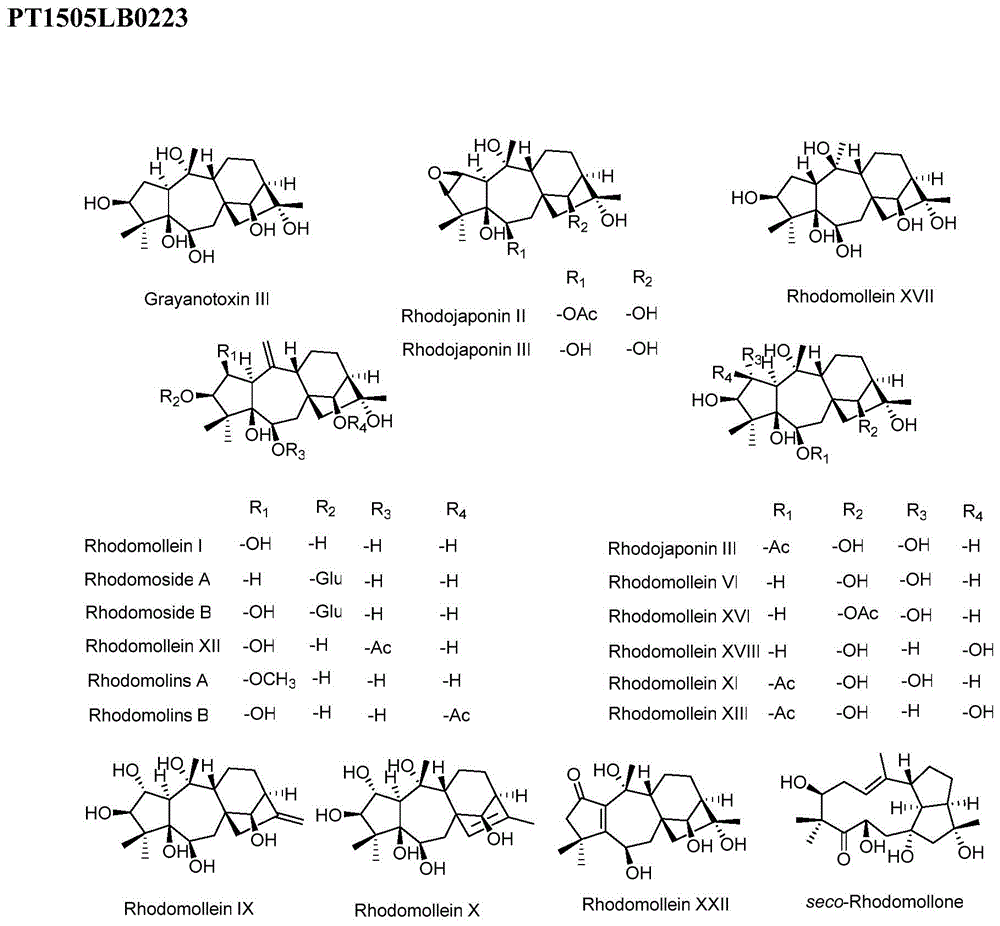
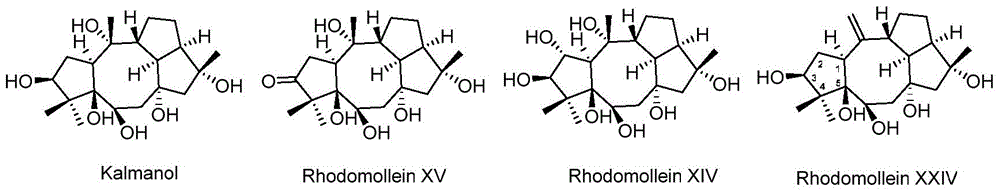
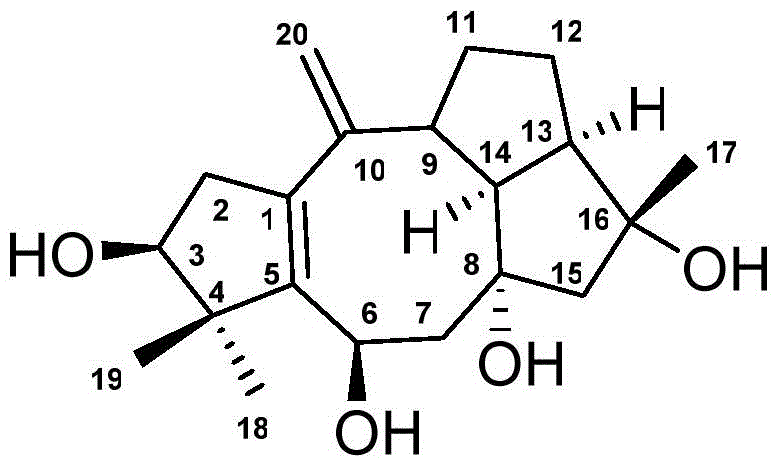
Anti-tumor drug of diterpenoid compound, and preparation method and application thereof
A technology for anti-tumor drugs and compounds, which is applied in the directions of anti-tumor drugs, separation/purification of hydroxy compounds, drug combination, etc., can solve the problems such as no reports on the activity of kalmanol-type diterpene components and few studies on cytotoxic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] One embodiment of the present invention provides a kind of diterpenoid compound, and its preparation method comprises:
[0037] A. Degrease the ramie flower with dichloromethane one or more times, filter, and collect the filter residue;
[0038] B. Extracting the syringa flower with alcohol or alcohol solution for one or more times, filtering, collecting the filtrate, concentrating and drying under reduced pressure, and obtaining the alcohol extract;
[0039] C. Add water to the alcohol extract, extract with petroleum ether and degrease again, then extract with ethyl acetate, then extract the obtained ethyl acetate extract with saturated aqueous sodium bicarbonate solution, take the ethyl acetate phase and evaporate to dryness to obtain ethyl acetate crude extract;
[0040] D. Purifying the obtained crude product by column chromatography to obtain purified diterpenoids.
[0041] The detailed steps of said step D may include:
[0042] ① The crude product is subjected ...
Embodiment 1
[0082] Naoyanghua (produced in Hubei) was taken, extracted three times with dichloromethane to degrease, filtered, and the filter residue was collected. Extract the filter residue three times with 95% ethanol solution, filter, collect the filtrate, and concentrate under reduced pressure to obtain the ethanol extract. Add water to the ethanol extract to make an extract aqueous suspension, extract three times with petroleum ether, degrease again, extract three times with ethyl acetate, concentrate the obtained ethyl acetate extract, then extract three times with saturated aqueous sodium bicarbonate solution, take acetic acid Ethyl phase was evaporated to dryness to obtain crude ethyl acetate extract.
[0083] The crude product was subjected to column chromatography on silica gel and eluted with ethyl acetate-ethanol gradient to obtain ethyl acetate-ethanol (volume ratio 30:1) eluate, which was concentrated to obtain extract E1. The extract E1 was subjected to silica gel column ...
Embodiment 2
[0085] Take 20Kg of Nao Yang Hua (produced in Hubei), extract three times with dichloromethane to degrease (60L×3), filter, and collect the filter residue. The filter residue was extracted three times with 95% ethanol solution (60L×3), filtered, the filtrate was collected, and concentrated under reduced pressure to obtain the ethanol extract (1.2Kg). Add 10L of water to the ethanol extract to make an extract aqueous suspension, extract three times with petroleum ether and degrease again (10L×3), extract three times with ethyl acetate (10L×3), and concentrate the obtained ethyl acetate extract to about 10 L was extracted three times with saturated aqueous sodium bicarbonate solution (10 L×3), and the ethyl acetate phase was evaporated to dryness to obtain ethyl acetate extract (crude product, about 200 g).
[0086] The crude product (about 200 g) was subjected to column chromatography on silica gel (600 g), eluted with ethyl acetate-ethanol gradient to obtain ethyl acetate-etha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com