Pharmaceutical composition containing pradaxa mesylate and preparation method thereof
A technology of dabigatran etexilate mesylate and composition, which is applied in the field of pharmaceutical composition containing dabigatran etexilate mesylate and its preparation, and can solve the problem of crystal form transformation of active substances, complex process, and environmental problems. pollution and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
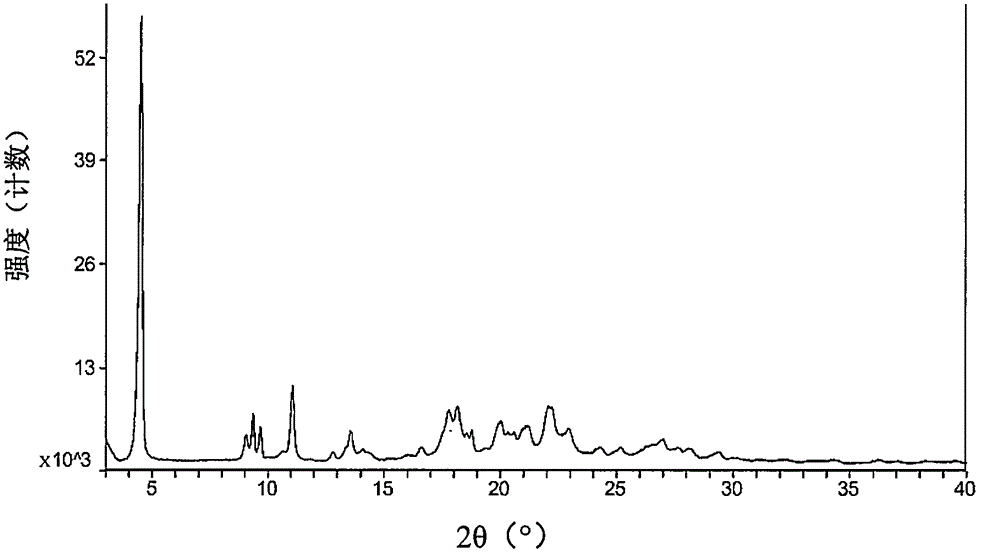
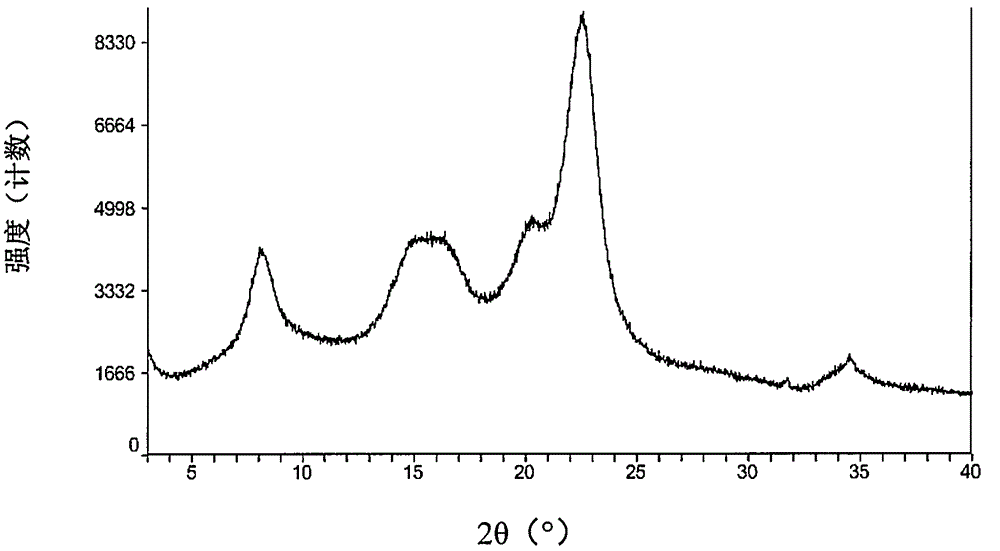
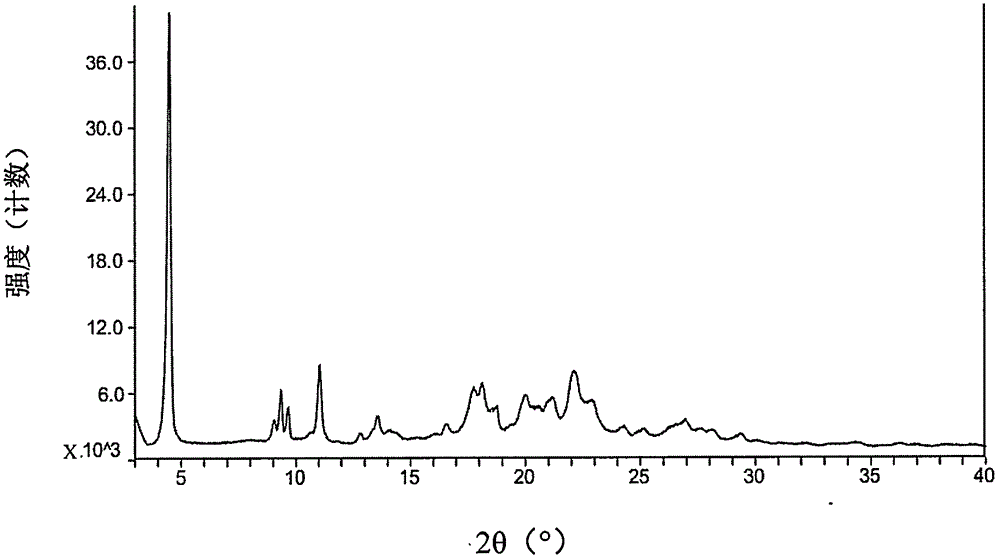
[0074] The starting material dabigatran etexilate mesylate form I can be prepared according to the method disclosed in Example 1 of WO2005028468A1.
[0075] The specific operation is: add 293 grams of acetone to 52.6 grams of dabigatran etexilate, and heat to 42° C. under stirring to form a clear solution. Afterwards, filter into a second stirring device followed by cooling to 33°C. Prepare 33 grams of acetone at 2° C., 7.9 grams of 99.5% methanesulfonic acid and 9 grams of acetone for rinsing, at 30° C. within 25 minutes, add to the solution of dabigatran etexilate with distribution metering, mixture 30 ℃ for 60 minutes, then cooled to 20 ℃ and stirred for 60 minutes. The crystal suspension was filtered, washed with 270 mL of acetone, and dried in vacuum at a maximum temperature of 50° C. for at least 4 hours to obtain crystalline form I of dabigatran etexilate mesylate.
Embodiment 1
[0077] According to the preparation method of the present invention, the pharmaceutical composition of the present invention using dabigatran etexilate methanesulfonate crystal form I as the active substance is prepared, and the formula and preparation method of each step are as follows.
[0078] (1) Preparation of Active Material Ball Core
[0079]
[0080] The preparation method of the active substance pellet core of No. 1a: Weigh 5g of hydroxypropyl methylcellulose, slowly add it into 20g of 80°C purified water under stirring, let it stand and cool to room temperature, dissolve the hydroxypropylmethylcellulose, and prepare into a binder solution. Put 85g of dabigatran etexilate mesylate mesylate crystal form I and 10g of microcrystalline cellulose into a wet granulator, mix well, and then add the above-mentioned prepared binder solution to make a suitable soft material. The soft material is extruded through a 0.6mm sieve and then rounded to obtain roughly spherical part...
Embodiment 2
[0102] (1) Preparation of active material ball core:
[0103] components
Weight (g)
Dabigatran etexilate mesylate crystal form I
70
25
Hydroxypropylmethylcellulose
5
10
Total (dry weight)
100
[0104] Weigh 5g of hydroxypropylmethylcellulose, slowly add it into 10g of 80°C purified water with stirring, let it stand and cool to room temperature, dissolve the hydroxypropylmethylcellulose, and prepare a binder solution. Put 70g of dabigatran etexilate methanesulfonate crystal form I and 25g of microcrystalline cellulose into a wet granulator, mix well, and then add the above-mentioned prepared binder solution to make a suitable soft material. The soft material is granulated through a 20-mesh sieve, and the prepared granules are dried in a fluidized bed at a material temperature of 30-35°C for 8-10 hours until the moisture content of the granules is less than 1%, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com