Preparation and medical application of stevanate a and its derivatives
A kind of alkaloid ester and fenestrate technology are applied in the application field of preparing antitumor drugs, and can solve the problems of no research report on the chemical composition of alkaloid, no record of medicinal use of alkaloid, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
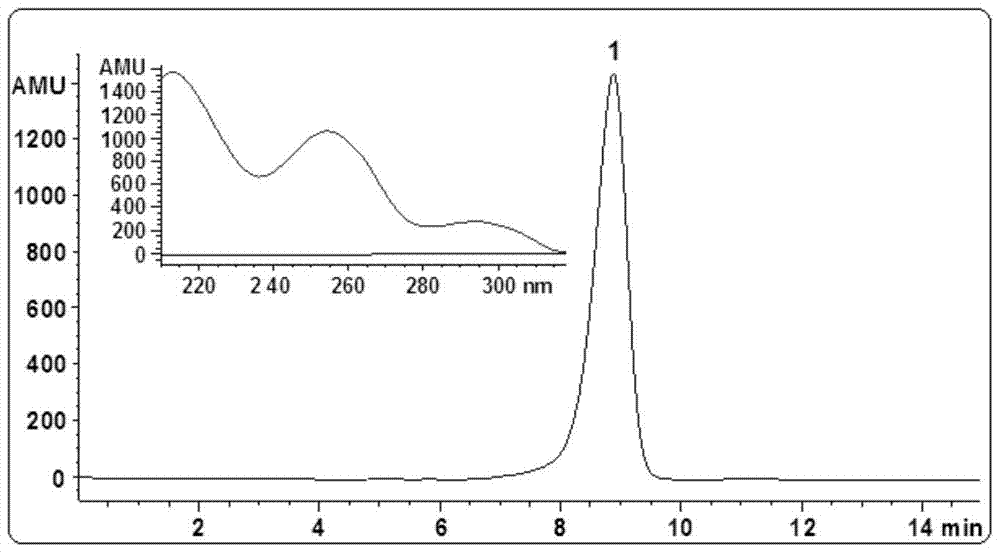
[0067] Example 1 Extraction and separation from plants to prepare asteryl asterate A
[0068] (1) Extraction, separation and purification of stevanate A:
[0069] Cut the whole herb of dried Aster asteroides into small sections and pulverize it into powder (1.5 kg) with a traditional Chinese medicine pulverizer, extract 3 times with methanol percolation (5000 milliliters for 12 hours for the first time, and 4000 milliliters for 5 hours each for the other times), The methanol percolation solution was combined and then concentrated under reduced pressure to obtain concentrated methanol extract (1000 ml). The concentrated methanol extract was extracted 3 times with cyclohexane (500 ml each time), and the combined cyclohexane extract was decompressed. Concentration gave cyclohexane extract extract (12.5 g). The cyclohexane extract extract was separated by silica gel column chromatography, and eluted sequentially with cyclohexane / ethyl acetate (9:1 and 4:1, v / v), and cyclohexane / e...
Embodiment 2
[0072] Example 2 Chemical Synthesis Preparation of Stennate A and Derivatives of Stennyl A
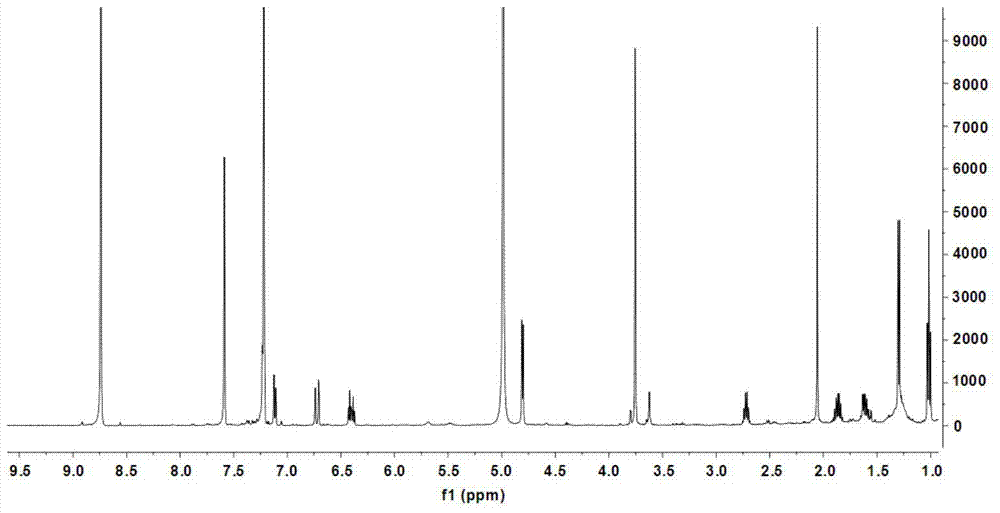
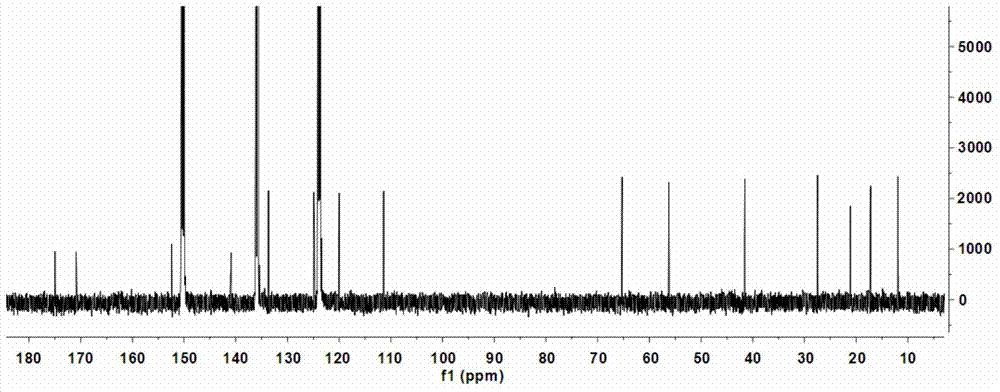
[0073] (1) Preparation of coniferic acid (3): 36.51 grams of vanillin (3a, 0.24mol), 31.22 grams of malonic acid (3b, 0.30mol), 60mL of dioxane, 30mL of anhydrous pyridine, and 3mL of aniline , keep the reaction at 90-95°C for 3 hours, cool to room temperature, add 200mL of 20% potassium carbonate and stir, adjust the pH to 2-3 with concentrated hydrochloric acid, precipitate the precipitate, filter it with suction, wash the solid with cold water, and dry it in vacuum Obtain coniferic acid (3,41.96 g) in the form of pale yellow-white powder. Molecular formula: C 10 h 10 o 4 ; 1 H-NMR data (500MHz, methanol-d 4 ): δ7.13(1H,d,J=1.9Hz,H-2),7.03(1H,dd,J=8.2 / 1.9Hz,H-6),6.80(1H,d,J=8.2Hz,H -5),7.57(1H,d,J=15.9Hz,H-7),6.28(1H,d,J=15.9Hz,H-8),3.86(3H,s,OCH 3 -3); HRESIMS m / z[M+Na] + 217.0452 (calculated value C 10 h 10 NaO 4 ,217.0477).
[0074] (2) Preparation of methyl coniferate...
Embodiment 3
[0097] Example 3 Inhibitory effect of stevanate A and its derivatives on the proliferation of glioma cells
[0098]Human glioma U87-MG and U251 cells were cultured with DMEM and 10% FBS medium in an incubator at 37°C and 5% carbon dioxide. Intestinal cancer HCT-15 cells were cultured with RPMI-1640 medium in an incubator at 37°C and 5% carbon dioxide, while intestinal cancer SW620 cells were cultured in a 37°C incubator with Leibovitz's L15 medium. All cells cultured for three generations were used for the experimental research of the present invention, and doxorubicin (DOX) and Temozolomide (TMZ), the first-line drug currently clinically used to treat glioma, were used as positive controls.
[0099] The survival rate of tumor cells was determined by the sulforhodamine B method (SRB). Cells were seeded in 96-well plates, and different concentrations of test drugs were added 24 hours after adherence. After 72 hours of drug treatment, stain with SRB, measure the absorbance at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com